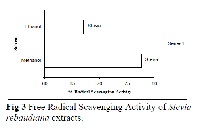
Efficient free radical scavenging activity of Ginkgo biloba, Stevia rebaudiana and Parthenium hysterophorous leaves through DPPH (2, 2-diphenyl-1-picrylhydrazyl)
Keywords:
Ginkgo biloba, Stevia rebaudiana and Parthenium hysterophorous, DPPH, radical scavenging activityAbstract
Free radical scavenging activity of three important plants Ginkgo biloba, Stevia rebaudiana and Parthenium hysterophorous was carried out to evaluate and explore new potential sources of natural antioxidants. For this purpose the leaves of the three plants were processed. In these experiments the order of the antioxidant activity was, maximum activity shown by methanolic extract of Ginkgo biloba followed by Parthenium hysterophorous and Stevia rebaudiana. Furthermore the ethanolic extract of Ginkgo biloba also showed maximum antioxidant activity seconded by Stevia rebaudiana and Parthenium hysterophorous.
References
Khanavi M, Hajimahmoodi M, CheraghiNiroomand M, Kargar Z, Ajani Y,
Hadjiakhoondi A, Oveisi MR. Comparison
of the antioxidant activity and total phenolic
contents in some Stachys species. Afr. J.
Biotechnol., 2009; 8, 1143-1147.
Huda-Faujan N, Noriham A, Norrakiah AS,
Babji AS. Antioxidant activity of plants
methanolic extracts containing phenolic
compounds. Afr. J. Biotechnol., 2009; 8,
-489.
Ramarathnam N, Osawa T, Ochi H,
Kawakishi S. The contribution of plant food
antioxidants to human health. Trends Food
Sci. Technol., 1995; 6: 75-77.
Demo A , Kefalas P, Boskou D. Nutrient
antioxidants in some herbs and
Mediterranean plant leaves. Food Res.
Int.,1998; 32, 351–354.
Sanchez-Moreno C, Larrauri JA, SauraCalixto F. Free radical scavenging capacity
and inhibition of lipid oxidation of wines,
grape juices and related polyphenolic
constituents. Food Res. Int., 1999; 32, 407-
Grice HC. Safety evaluation of butylated
hydroxytoluene (BHT) in the liver, lung and
gastrointestinal tract. Food Chem. Toxicol.,
; 24, 1127-1130.
Gulcin ME, Buyukokuro L, Oktay M,
Kufrevio O lu. On the in vitro antioxidant
properties of melatonin. J. Pineal Res., 2002;
, 167-171.
Gulcin ME, Mshvildadze V, Gepdiremen
A, Elias R a. Screening of antioxidant and
antiradical activity of monodesmosides and
crude extract from Leontice smirnowii
Tuber. Phytomedicine, 2006; 20,130-134.
Cook NC, Samman S. Flavonoids:
chemistry, metabolism, cardioprotective
effects and dietary sources. J. Nut.
Biochem., 1996; 7, 66-76.
Liyana-Pathirana CM, Shahidi F.
Antioxidant properties of commercial soft
and hard winter wheats (Triticum aestivum
L.) and their milling fractions. J. Sci. Food
Agric., 2006; 86: 477-485.
Ames BN, Shigenaga MK, Hagen TM.
Oxidants, antioxidants and the generative
disease of aging. Proc. Natl. Acad. Sci.,
USA 1993; 90, 7915-7922.
Dean RT, Davies MJ. Reactive species and
their accumulation on radical damaged
proteins. Trends Biochem. Sci., 1993; 18,
-441.
Ceruti P. Oxy-radicals and cancer.
Lancet,1994; 344, 862-863.
Tutour BL. Antioxidative activities of algal
extracts. Synergistic effect with vitamin E.
Phytochem., 1990; 29, 3759-3765.
Bronen AL. Toxicology and biochemistry of
butylated hydroxy anizole and butylated
hydroxy toluene. J. Am. Oil. Chem. Soc.,
; 52, 59-63.
Couladis M, Tzakou O, Verykokidou E.
Screening of some Greek aromatic plants for
antioxidant activity. J. Phytother. Res.,
; 17, 194-196.
Linn CC, Wu SJ, Chang Ch. Antioxiant
activity of Cinammomum cassia. J.
Phytother. Res., 2003; 17, 726-730.
Fukuda Y, Osawa T, Namiki M. Studies on
antioxidant substance in sesame seeds.
Agric. Biol. Chem., 1990; 49: 301-306.
Igarashi K, Itoh M, Harada T. Major
antioxidtive substances in leaves AtsumiKabu (Red turnip, Brassica comoestris L.)
Agric. Biol. Chem., 1990; 54, 1053-1055.
Evelson P, Travacio M, Repetto M.
Evaluation of total reactive antioxidant
potential of tissue homogenates and their
cytosols. Archives Biochemistry and
Biophysics, 2001; 388, 261–266.
Van den Berg R, Haenen GRMM, Van den
Berg H. Applicability of an improved trolox
equivalent antioxidant capacity assay for
evaluation of antioxidant capacity
measurements of mixtures. Food Chemistry,
; 66, 511–517.
Cao G, Prior RL. Measurement of oxygen
radical absorbance capacity in biological
samples. Methods in Enzymology,1999;
, 50–62.
Ou B, Hampsch-Woodill M, Prior RL.
Development and validation of an improved
oxygen radical absorbance capacity assay
using fluorescein as the fluorescent probe.
Journal of Agriculture and Food Chemistry,
; 49, 4619–4626.
Benzie IFF, Strain JJ. The ferric reducing
ability of plasma as a measure of
‘‘antioxidant power’’: the FRAP assay.
Analytical Biochemistry, 1996; 239, 70–76.
Shinozuka K, Umegaki K, Kubota Y,
Tanaka N. Feeding of Ginkgo biloba extract
(GBE) enhances gene expression of hepatic
cytochrome P-450 and attenuates the
hypotensive effect of nicardipine in rats.
Life Sci., 2002; 70, 2783-2792.
Jarvis AP, Morgan D. Isolation of plant
products by supercritical-fluid extraction.
Phytochem Anal., 1997; 8, 217-222.
Oyama Y, Fuchs PA, Katayama N, Noda K.
Myricetin and quercetin, the flavonoid
constituents of Ginkgo biloba extract,
greatly reduce oxidative metabolism in both
resting and Ca2+ loaded brain neurons. Brain
Res., 1994; 635, 125-129.
Kleinjen J, Knipschild P. Ginkgo biloba -
drug profile. Lancet, 1992; 340, 1136-8.
Apaydin C, Oguz Y, Agar A, Yargicoglu P,
Demir N, Aksu G. Visual evoked potentials
and optic nerve histopathology in normal
and diabetic rats and effect of Ginkgo biloba
extract. Acta Ophthalmol Copenh, 1993; 71,
-628.
Therond P, Rousselot D, Spraul A, Conti
M, Legrand A. Biomarkers of oxidative
stress: An analytical approach. Cur.r Opin.
Clin. Nutr. Metab., Care 2000; 3, 373–384.
Marcocco L, et al. Antioxidant action of
Ginkgo biloba extracts Egb 761. Methods
Enzymol., 1994; 234, 462-475.
Maitra I, et al. Peroxyl radical scavenging
activity of Ginkgo biloba extract Ebb 761.
Bilchem Pharmacol., 1995; 49(11), 1649-
Hasler A, Sticher O, Meier B.
Identification and determination of the
flavonoids from Ginkgo biloba by highperformance liquid chromatography. J.
Chromatogr., 1992; 605, 41-48.
Mantle et al. Comparison of Antioxidant
Activity in Commercial Ginkgo biloba
Preparations. J. of Alternative and
Complementary Medicine, 2003; 9, 5, pp.
–629.
Liu JC, Kao PK, Chan P, Hsu YH, Hou
CC, Lien GS, Hsieh MH, Chen YJ, Cheng
JT. Mechanism of the antihypertensive
effect of stevioside in anesthetized dogs.
Pharmacology 2003; 67(1):14.
Soejarto DD, Kinghorn AD, Farnsworth NR.
Potential sweetening agents of plant origin.
III. Organoleptic evaluation of Stevia leaf
herbarium samples for sweetness. J Nat
Prod., 1982; 45(4), 590.
Hanson JR, Oliveira BH. Stevioside and
related sweet diterpenoid glycosides. Nat.
Prod. Rep., 1993; 10, 301–309.
Gregersen S, Jeppesen PB, Holst JJ,
Hermansen K. Antihyperglycemic effects of
stevioside in type 2 diabetic subjects.
Metabolism, 2004; 53(1):73.
Benford DJ, DiNovi M, Schlatter J. Safety
Evaluation of Certain Food Additives:
Stevio Glycosides. WHO Food Addit. Ser.,
; 5, 117–144.
Chan P, Linson B, Chen Y, Liu J, Hsieh
M, Cheng J. A double blind placebocontrolled study of the effectiveness and
tolerability of oral stevioside in human
hypertension. Br. J. Clin. Pharmacol., 2000;
, 215–220.
Lee CN, Wong K, Lin J, Chen Y, Chen J,
Chan P. Inhibitory effect of stevioside on
calcium influx to produce antihypertension.
Planta Med., 2001; 67, 796– 799.
Suanarunsawat T, Chaiyabutr N. The effect
of stevioside on glucose metabolism in rat.
Can. J. Physiol. Pharmacol., 1997; 75, 976–
Jutabha PC, Chatsudthipong V. Effect of
stevioside on PAH transport by isolated
perfused rabbit renal proximal tubule. Can.
J. Physiol. Pharmacol., 2000; 78, 737–744.
Melis MS. Chronic administration of
aqueous extract of Stevia rebaudiana in rats:
renal effects. J. Ethnopharmacol., 1995; 47,
–134.
Buran P, Supak P. Antioxidant Capacities of
Pueraria mirifica, Stevia rebaudiana
Bertoni, Curcuma longa Linn.,
Andrographis paniculata (Burm.f.) Nees.
and Cassia alata Linn. for the Development
of Dietary Supplement Kasetsart J. (Nat.
Sci.) 2007; 41, 548 – 554.
Shukla. In vitro antioxidant activity and total
phenolics content of ethanolic leaf extract of
Stevia rebaudiana Bert. Food and Chemical
Toxicology, 2009; 47, 2338–2343.
Haseler WH. Parthenium hysterophorus L.
in Australia. PANS 1976; 22, 515-517.
Dale IJ. Parthenium weed in the Americas.
Aust. Weeds,1981; 1, 8-14.
Rao RS. Parthenium a new record for India.
J. Bombay Nat. Hist. Soc 1956; 54: 218-
Butler JE,. Longetivity of Parthenium
hysterophorus L. seed in the soil.
Aust.Weeds. 1984; 3:6.
Brand WW, Cuvelier HE, Berset C. Use of
a free radical method to evaluate antioxidant
activity, Food Science and Technology,
; 25–30.
Halliwell B. Oxidative stress, nutrition and
health. Experimental strategies for
optimization of nutritional intake in humans.
Free Radic Res., 1996; 25, 57–74.icol Pathol
; 31: 682-688.