Chemopreventive effects of pomegranate fruit extract on acrylamide induced lung, liver and testis carcinogenesis in male laka mice
Keywords:
Acrylamide, Carcinogenesis, Catalase, Glutathione peroxidase, Glutathione-S-transferase, Pomegranate Fruit Extract, Superoxide dismutaseAbstract
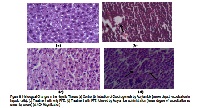
Objective: The present study was aimed to test the efficacy of pomegranate fruit extract to induce apoptosis in the artificially induced tumor cells in mice. Method: Adult male LAKA-UK mice (30-40g) were divided into four groups, viz. control, AA, PFE and PFE+AA. Induction of carcinogenesis by intra -peritoneal administration of acrylamide was preceded by PFE treatment in PFE+AA group. At the end of study animals were sacrificed by decapitation under deep anesthesia and organs (lung, liver and testes) were resected to evaluate activities of various enzymes such as CAT, SOD, GPx, GSH and GST in different experimental groups. MTT assay and histological examination of lung and liver sections were also conducted. Result: Administration of acrylamide resulted in the increase SOD, GPx and GSH activities, which decreased significantly (p<0.05) in PFE+AA group. The MTT assay showed high cell proliferation in the AA group of mice which lessened in treatment groups. Similarly the histological examinations exhibited alveolar wall destruction and air space enlargement in pulmonary tissues and larger vacuolization in the hepatic tissues due to acrylamide administration, whereas in PFE+AA cells it was found to be normal. Conclusion: Pomegranate fruit extract was observed to be a potential treatment intervention that not only prevents the onset and progress of carcinogenesis but also helps in the initiation of apoptosis and refurbishment of the damaged cellular architecture.
References
Beckett WS. Epidemiology and etiology of lung cancer. Clin Chest Med 1993; 14: 1-15.
László H, Zsolt T. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci 2010; 14: 249-258.
Drain L. Testicular cancer in California from 1942–1969: The California Tumor Registry experience. Oncology 1973; 27: 45–51.
Maffezzini M. TC Incidence Increasing: Spread the Word. Eur Urol 2007; 51: 596–597.
Agrawal AK, Seth PK, Squibb RE, Tilson HA, Uphouse LL, Bondy SC. Neurotransmitter receptors in brain regions of acrylamide- treated rats: I. Effects of a single exposure to acrylamide. Pharmacol Biochem Behav 1981; 14: 527–31.
Johnson KA, Gorzinski SJ, Bodner KM, Campbell RA, Wolf CH, Friedman MA, et al. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol Appl Pharmacol 1986; 85(2): 154–168.
Bull RJ, Robinson M, Laurie RD, Stoner GD, Greisiger E, Meier JR, et al. Carcinogenic effects of acrylamide in Sencar and A/J mice. Cancer Res 1984; 44(1): 107–111.
Bull RJ, Robinson M, Stober JA . Carcinogenic activity of acrylamide in the skin and lung of Swiss-ICR mice. Cancer Lett 1984; 24 (2): 209 – 212.
Friedman MA, Dulak LH, Stedham MA. A lifetime oncogenicity study in rats with acrylamide . Fundamen Appl Toxicol 1995; 27 (1): 95–105.
Song-Tay L, Min-Hua L, Lan-Hsiang C, Ting-Feng W, Li-Chien H, Gwo-Ing L. Suppression of urinary bladder urothelial carcinoma cell by the ethanol extract of pomegranate fruit through cell cycle arrest and apoptosis. BMC Compl Alter Med 2013; 13: 364.
Cerda B, Ceron JJ, Tomas-Barberan FA, Espin JC: Repeated oral administration of high doses of pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J Agri Food Chem 2003; 51: 3493–3501.
Cerda B, Llorach R, Ceron JJ, Espin JC, Tomas-Barberan FA: Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr 2003; 42: 18–28.
Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA: Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agri Food Chem 2000; 48: 4581–4589.
Hong MY, Seeram NP, Heber D: Pomegranate polyphenols downregulate expression of androgen synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. J Nutr Biochem 2008; 19: 848–855.
Aviram M, Dornfeld L, Kaplan M, Coleman R, Gaitini D, Nitecki S et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: studies in atherosclerotic mice and in humans. Drugs Exp Clin Res 2002; 28(2-3): 49-62.
Lippman SM, Lee JJ, Sabichi AL. Cancer chemoprevention: progress and promise. J Nat Cancer Inst 1998; 90: 1514–1528.
Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanins- and hydrolysable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathway and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer 2005; 113: 423–433.
Kawaii S, Lansky EP. Differentiation-promoting activity of pomegranate (Punica granatum) fruit extracts in HL-60 human promyelocytic leukemia cells. J Med Food 2004; 7: 13–18.
Khan N, Adhami VM, Mukhtar H. Apoptosis by dietary agents for prevention and treatment of prostate cancer. Endocr Relat Cancer 2010; 17(1): R39–R52.
Adams LS, Seeram NP, Agarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins and punicalagin suppress inflammatory cell signalling in colon cancer cells. J Agri Food Chem 2006; 54: 980-985.
Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol 2006; 6: 532–40.
Luck H. Catalase In: Methods of Enzymatic Assays (Ed): HU Bergmeyer. New York, Academic Press. 1965; 885-894.
Kono Y, Fridovich I. Superoxide radicals inhibit catalase. J Biol Chem 1982; 257: 5751-5754.
Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979; 582: 67–78.
Paglia DE, and Valentine WN. Studies on the quantitative and qualitative characterization of glutathion proxidase. J Lab Med 1987; 70: 158-165.
Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol 1981; 77: 398–405.
Lowry O, Rosebrough A, Far A, Randall R. Protein measurement with folin phenol reagent. J Biol Chem 1951;
: 680-685.
Supine R. MTT assay. In the ERGATT/FRAME Data Bank. In vitro Techniques in Toxicology 1990; 17.
Dahl GA, Miller JA, Miller EC (1978). Vinyl carbamate as a promutagen and a more carcinogenic analog of ethyl carbamate. Cancer Res 1978; 38: 3793- 3804.
Nakamura Y, Ohigashi H, Masuda S et al. (2000). Redox regulation of glutathione-S-transferase induction by benzyl isothiocynate: correlation of enzyme induction with the formation of reactive oxygen intermediates. Cancer Res 2000; 60: 219-225.
Sumner SC, Williams CC, Snyder RW, Krol WL, Asgharian B, Fennell TR. Acrylamide: a comparison of metabolism and haemoglobin adducts in rodentsfollowing dermal, intraperitoneal, oral or inhalation exposure. Toxicol Sci 2003; 75: 260-70.
Qunying X, Ye L, Hongfang S. Inhibition of acrylamide toxicity in mice by three dietary constituents. J Agri Food Chem 2008; 56: 6054-6060.
Yousuf MI and El-Demerdash. Acrylamide induced oxidative stress and biochemical perturbations in rats. Toxicol 2006; 219: 133-141.
Schwartz JL, Tanaka J, Khandekar V, Herman TS, Teicher B.. β-Carotene and/or vitamin E as modulators of alkylating agents in SCC-25 human squamous carcinoma cells. Chemother Clin Pharm 1992; 50: 367-373.
Balasenthil S, Nagini S, Arivazhagan S. Reactive oxygen species oxidative damage and pathogenesis. Current Sci 1999; 77(5): 658-65.
Palozza P, Calviello G, De Leo ME, Serini S, Bartoli GM. Canthaxanthin supplementation alters antioxidant enzymes and iron concentration in liver of Balb/c mice. J Nutri 2000; 130: 1303-1308.
Chance B, Sies H, Boveris A. Hydrogen peroxide mechanism in mammalian organs. Physiol Rev 1979; 59: 527-605.
Yu BP. Cellular defences against damages from reactive oxygen species. Am Physiol Soc 1994; 139-62.
Oberley LW, Oberley TD. Free radicals and aging. In free radicals, ageing and degenerative diseases. Johnson JE, Walford R Jr., Harmon D, Maguel J, editors. New York Liss 1986; 325-71.
Helen A, Vijayammal PL. Effects of Vitamin A supplementation on cigarette smoke induced lipid peroxidation. Vet Hum Toxicol 1997; 39(1): 18-21.
Sridevi B, Reddy KV, Reddy SLN. Effect of trivalent and hexavalent chromium on antioxidant enzyme activities and lipid peroxidation in a freshwater field crab. Barytellphusa guerini. Bull Environ Contam Toxicol 1998; 61 : 384-390.
Kirkova M, Alexandro A, KEsiova M, Todorov S. In vivo effects of amtolmetin guacyl on lipid peroxidation and antioxidant defence systems. Comparisons with non-selective and COX-2 selective NSAIDS. Auton Autocoid Pharmacol 2007; 27(2): 99-104.
Wengen FA, Killian M, Bisevac M, Khodadayan C, von Seebach M, Schimke I et al. Effects of Colecoxib and Zyflo on liver metastasis and lipid peroxidation in pancreatic cancer in Syrian hamsters. Clin Exp Metastasis 2002; 19: 681-687.
Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis 2000; 21: 485-495.
Banerjee S, Segal A. In vitro transformation of C3H/ 10T1/ 2 and NIH/ 3T3 cells by acrylonitrile and acrylamide. Cancer Lett 1986; 32: 698-701.
Tsuda HS, Taketomi CS, Hasegawa MM, Hamada A, Katawa KM, Inui N. Acrylamide: induction of DNA damage and chromosomal aberrations and cell transformation without gene mutations. Mutagenesis 1993; 8: 23-29.
Liaw L, Schwartz SM. Microtubule disruption stimulates DNA synthesis in bovine endothelial cells and potentiates cellular response to basic fibroblast growth factor. Am J Pathol 1993; 143: 937-948.
Pienta KJ, Coffey DS. Nuclear-cytosketal interaction: evidence for physical connections between nucleus and cell periphery and their alteration by transformation. J Cell Biochem 1992; 49: 357-365.
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003; 362: 1907-1917.