Phytochemical Screening and In-Silico Investigation of 2-Benzoxazolinone from Acanthus Ilicifolius Linn. as Dual Inhibitors of Cyclooxygenase-2 and 5-Lipooxygenase Enzymes
Keywords:
Supercritical CO2 fluid extract, Acanthus ilicifolius Linn, UHPLC, Mangroves, COX-2/5-LOX, Genetic Optimization for Ligand DockingAbstract
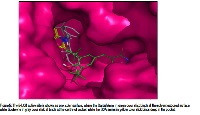
2-Benzoxazolinone (BOA) is a phytoconstituent of a mangrove plant Acanthus ilicifolius Linn used to treat inflammatory diseases. Anti-inflammatory agents with dual inhibiting properties of COX-2/5-LOX enzymes activities are said to possess anti-inflammatory effect and are devoid from side effects but not yet available on the market. Therefore, the dual inhibiting property of BOA and its derivatives [6-Bromo-BOA (6-BrBOA), 6-Chloro-BOA (6-ClBOA), Hydroxy-BOA (HBOA) and 6-methoxy BOA (6-MBOA)] is worth investigating. Initially, BOA content in supercritical CO2 fluid extract (SCFE) of A.ilicifolius leaves was estimated using ultra high performance liquid chromatography (UHPLC). Later, genetic algorithm based GOLD docking simulation was employed to dock BOA and its derivatives into the binding pocket of COX-2 and allosteric binding site of 5-LOX. Probable hit ligand was ranked using an evaluation criterion based on the GOLD docking score and the ligand binding mode. The UHPLC chromatogram revealed the presence of BOA in SCFE. High gold docking scores ranging from 40.89 to 43.13 were observed in all the tested ligands with COX-2 and three ligands namely; 6-BrBOA (42.67), 6-ClBOA (41.79) and BOA (38.90) showed high docking scores with 5-LOX allosteric binding site. In conclusion, UHPLC method could be used for the determination of BOA content in A.ilicifolius. BOA and its analogues could be a promising lead for developing dual inhibitors of COX-2/5-LOX enzymes as verified by computational studies.
References
A. P. Athira, A. Helen, K. Saja, P. Reddanna and P. R. Sudhakaran, "Inhibition of Angiogenesis In Vitro by Chebulagic Acid: A COX-LOX Dual Inhibitor," Int J Vasc Med, vol. 2013, pp. 843897, 2013.
S. Fiorucci, R. Meli, M. Bucci and G. Cirino, "Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy?," Biochem Pharmacol, vol. 62, no. 11, pp. 1433-1438, 2001.
F. Julemont, J. M. Dogne, B. Pirotte and X. de Leval, "Recent development in the field of dual COX / 5-LOX inhibitors," Mini Rev Med Chem, vol. 4, no. 6, pp. 633-638, 2004.
J. M. Dogne, J. Hanson, C. Supuran and D. Pratico, "Coxibs and cardiovascular side-effects: from light to shadow," Curr Pharm Des, vol. 12, no. 8, pp. 971-975, 2006.
J. Kaur, A. Bhardwaj, Z. Huang and E. E. Knaus, "Aspirin analogues as dual cyclooxygenase-2/5-lipoxygenase inhibitors: synthesis, nitric oxide release, molecular modeling, and biological evaluation as anti-inflammatory agents," ChemMedChem, vol. 7, no. 1, pp. 144-150, 2012.
R. N. Ram and V. K. Soni, "Synthesis of 3-alkylbenzoxazolones from N-alkyl-N-arylhydroxylamines by contiguous O-trichloroacetylation, trichloroacetoxy ortho-shift, and cyclization sequence," J Org Chem, vol. 78, no. 23, pp. 11935-11947, 2013.
N. Gokhan-Kelekci, M. Koksal, S. Unuvar, G. Aktay and H. Erdogan, "Synthesis and characterization of some new 2(3H)-benzoxazolones with analgesic and antiinflammatory activities," J Enzyme Inhib Med Chem, vol. 24, no. 1, pp. 29-37, 2009.
R. Wöstmann and G. Liebezeit, "Chemical composition of the mangrove hollyAcanthus ilicifolius (Acanthaceae) — review and additional data," Senckenbergiana maritima, vol. 38, no. 1, pp. 31-37, 2008.
L. Liu, H. Fan, P. Qi, Y. Mei, L. Zhou, L. Cai, X. Lin and J. Lin, "Synthesis and hepatoprotective properties of alkaloid A and its derivatives," Exp Ther Med, vol. 6, no. 3, pp. 796-802, 2013.
S. Ravikumar, M. Raja and M. Gnanadesigan, "Antibacterial potential of benzoate and phenylethanoid derivatives isolated from Acanthus ilicifolius L. leaf extracts," Nat Prod Res, vol. 26, no. 23, pp. 2270-2273, 2012.
T. Fornari, G. Vicente, E. Vazquez, M. R. Garcia-Risco and G. Reglero, "Isolation of essential oil from different plants and herbs by supercritical fluid extraction," J Chromatogr A, vol. 1250, pp. 34-48, 2012.
G. Chiapusio, F. Pellissier and C. Gallet, "Uptake and translocation of phytochemical 2-benzoxazolinone (BOA) in radish seeds and seedlings," J Exp Bot, vol. 55, no. 402, pp. 1587-1592, 2004.
A. Estella-Hermoso de Mendoza, M. A. Campanero, F. Mollinedo and M. J. Blanco-Prieto, "Comparative study of A HPLC-MS assay versus an UHPLC-MS/MS for anti-tumoral alkyl lysophospholipid edelfosine determination in both biological samples and in lipid nanoparticulate systems," J Chromatogr B Analyt Technol Biomed Life Sci, vol. 877, no. 31, pp. 4035-4041, 2009.
R. G. Kurumbail, A. M. Stevens, J. K. Gierse, J. J. McDonald, R. A. Stegeman, J. Y. Pak, D. Gildehaus, J. M. Miyashiro, T. D. Penning, K. Seibert, P. C. Isakson and W. C. Stallings, "Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents," Nature, vol. 384, no. 6610, pp. 644-648, 1996.
N. C. Gilbert, S. G. Bartlett, M. T. Waight, D. B. Neau, W. E. Boeglin, A. R. Brash and M. E. Newcomer, "The structure of human 5-lipoxygenase," Science, vol. 331, no. 6014, pp. 217-219, 2011.
E. F. Pettersen, T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng and T. E. Ferrin, "UCSF Chimera--a visualization system for exploratory research and analysis," J Comput Chem, vol. 25, no. 13, pp. 1605-1612, 2004.
E. E. Bolton, Y. Wang, P. A. Thiessen and S. H. Bryant, "Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities," in Annual Reports in Computational Chemistry, A. W. Ralph and C. S. David, Ed., pp. 217-241, Elsevier, 2008.
M. L. Verdonk, J. C. Cole, M. J. Hartshorn, C. W. Murray and R. D. Taylor, "Improved protein–ligand docking using GOLD," Proteins: Structure, Function, and Bioinformatics, vol. 52, no. 4, pp. 609-623, 2003.
J. An, M. Totrov and R. Abagyan, "Pocketome via comprehensive identification and classification of ligand binding envelopes," Mol Cell Proteomics, vol. 4, no. 6, pp. 752-761, 2005.
G. M. Morris, D. S. Goodsell, R. S. Halliday, R. Huey, W. E. Hart, R. K. Belew and A. J. Olson, "Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function," Journal of Computational Chemistry, vol. 19, no. 14, pp. 1639-1662, 1998.
H. R. Bravo, S. V. Copaja and J. San Martin, "Contents of 1,4-benzoxazin-3-ones and 2-benzoxazolinone from Stenandrium dulce (Nees)," Z Naturforsch C, vol. 59, no. 3-4, pp. 177-180, 2004.
A. Madeswaran, M. Umamaheswari, K. Asokkumar, T. Sivashanmugam, V. Subhadradevi and P. Jagannath, Discovery of potential cyclooxygenase inhibitors using in silico docking studies, 2012.
A. Madeswaran, M. Umamaheswari, K. Asokkumar, T. Sivashanmugam, V. Subhadradevi and P. Jagannath, Docking studies: In silico lipoxygenase inhibitory activity of some commercially available flavonoids, 2012.