Isolation of 12 Bacterial endophytes from some mangrove plants and determination of, antimicrobial properties of the isolates and the plant extracts
Keywords:
Bacterial endphytes, Bacteriocins, Mangrove plants, AntimicrobialsAbstract
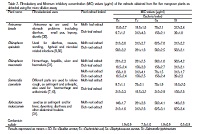
The mangrove designates a highly productive ecosystem with important economic and environmental functions. Endophytes are microorganisms that live in the intercellular spaces of plant tissue. This study aimed to isolate and identify bacterial endophytes from five mangrove plants and to determine, antimicrobial properties of the isolates and the plant extracts against four pathogenic bacteria: Bacillus cereus, Staphylococcus aureus, Escherichia coli and Salmonella typhimurium using the deferred antagonism and the microdilution assays. Of the total 33 endophytic bacteria isolated, 18 strains showed antagonistic effects. Twelve of these inhibitors were identified using VITEK 2. Crude protein from each of the producer strains were precipitated and tested for minimum inhibitory concentration (MIC) against the pathogenic bacteria using the microdilution assay. Best activities were recorded for Staphylococcus intermedius and Bacillus licheniformis (19 µl/ml) against B. cereus. The S. intermedius also inhibited growth of both S. aureus and S. typhimurium (39 µl/ml). Staphylococcus lentus, Bacillus pumilus and Bacillus coagulans possessed activities against S.typhimurium with an MIC value of 78 µg/ml. For the plant extract, the lowest MIC value (9.7 µg/ml) was obtained by Aviecenna lanata and Sonneratia caseolaris against B.cereus. S.caseolaris also showed significant inhibitory effects against E.coli and S.typhimurium (19.5 µg/ml). Our results indicated the potentiality of the isolated bacterial endophytes as a producer of antimicrobial substances which could be developed for various applications. MIC values obtained for the plant extracts in this study showed the effective plant part and extracts to be further developed and profiled as antimicrobial agents.
References
Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol biol R.2002; 67:491–502.
Bhimba BV, Franco,AD, Mathew JM, Jose GM, Joel EL, Thangaraj M. Anticancer and antimicrobial activity of mangrove derived fungi Hypocrea lixii VB1. Chinese.J.of N.Med. 2012; 10 (Suppl 1): 0077-0080.
Kayalvizhi N, Gunasekaran P. Purification and characterization of a novel broad-spectrum bacteriocin from Bacillus licheniformis MKU3. Biotechnol and Biop.Eng.2010;15:365-370.
Molina G, Pimentel MR, Bertucci TCP, Pastore GM. Application of fungal endophytes in biotechnological processes. Chem Eng.Transaction. 2012;27:289-294.
Altuntas E. Bacteriocins: A natural way to combat with pathogens In: Méndez-Vilas Ed. Microbial pathogens and Strategies for Combating them: Science, Technology and Education. FORMATEX Microbiology Book Series.Formatex Research Centre: Badajoz, Spain.; 2013. P.1005-1015.
Premanathan M, Arakaki R, Izumi H, Kathiresan K, Nakano M, Yamamoto N, Nakashima, H. Antiviral properties of a mangrove plant, Rhizophora apiculata against immunodeficiency virus. Antiviral Research. 1999;44:113-122.
Bandaranayake WM. Traditional and medicinal uses of mangroves Mangroves and Salt Marshes. 1998; 2: 133–148.
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: recent developments and applications.FEMS Microbial Letter. 2008; 278: 1-9.
Eldeen I MS, Effendy MAW. Antimicrobial agents from mangrove plants and their ndophytes. In: Méndez-Vilas Ed. Microbial pathogens and Strategies for Combating them: Science, Technology and Education. FORMATEX Microbiology Book Series.Formatex Research Centre: Badajoz, Spain.; 2013. P.872-882.
Bandaranayake WM. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetlands Ecology and Management. 2002; 10: 421–452.
Sittiwet C. In vitro antimicrobial activity of Pulchea indica aqueous extract: The potential for urinary infection treatment. J Pharmacol and Toxicol.2009;4:87-90.
Saad S, Taher M, Susanti D, Qaralleh H, Awang AF. In vitro antimicrobial activity of mangrove plant Sonneratia alba. Asian Pacific J Tropical Biomed. 2012; 427-429.
Arvuselvan N, Jagadeesan D, Govindan T, Kathiresan K, Anantharaman P. In vitro antibacterial activity of leaf and bark extracts of selected mangrove against fish and shrimp pathogens. Global J of Pharmacol 2011; 5:112-116.
Rouf R, Uddin, SJ, Shilpi JA, Alamgir M. Assessment of antidiarrhoeal activity of the methanol extract of Xylocarpus granatum bark in mice model. J Ethnopharmacol. 2007; 109: 539–542.
Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med.1998; 64:711-713.
Graf B, Adam T, Zill E, Göbel U.Evaluation of VITEK2 system for rapid identification of yeast and yeast-like organisms.J Clin Microbiol.2000; 38(Suppl 5):1782-1785.
Ling TKW, Liu Z K, Cheng AFB. Evaluation of the VITEK 2 system for rapid direct identification and susceptibility testing of Gram-negative Bacilli from positive blood cultures. J Clin Microbiol.2003; 41(Suppl 10): 4705-4707.
Alekseevna BI, Danilovich, KA, Valerievna KU. Antimicrobial activity of heterotrophic bacterial strains of marine origin. Jundishapur J of Microbiol.2013;2:166-175.
Riely MA, Chavan, MA. Bacteriocins:Ecology and Evolution. Berlin Heidelberg New York: Springer –Verlag; 2007:P.1-3.
Yilmaza M, Sorana H, Beyatlib, Y. Antimicrobial activities of some Bacillus sp. strains isolated from the soil. Microbiological Res.2006; 161: 127-131.
Béri T, Koji M, Stankovi S, Topisirovi L, Degrassi G, Myers M, Venturi V, Djord et al. Antimicrobial Activity of Bacillus sp. Natural isolates and their potential use in the biocontrol of phytopathogenic Bacteria. Food Technology Biotechnol. 2012;50 (Suppl 1): 25–31 .
Heng NC, Wescombe PA, Burton JP, Jack RW, Tagg JR.The Diversity of Bacteriocins in ram-Positive Bacteria. In: Riley MA, Chavan MA Ed: Bacteriocins: Ecology and Evolution. Springer-Verlag Berlin Heidelberg. . 2007; P.54-92.
Aslim B, Sağlamn N, Beyatli Y. Determination of some properties of Bacillus isolated from Soil. Turk J Biol. 2002; 26:41-48.
Gong H-S, Meng X-C, Wang H. Mode of action of plantaricin MG, a bacteriocin active against Salmonella typhimurium. J Basic Microbiol. 2010; 50:37- 45.
Kayalvizhi N, Gunasekaran P. Purification and characterization of a novel broad-spectrum bacteriocin from Bacillus licheniformis MKU3. Biotechnol Bioprocess Engineering.2010;15:365-370.
Smith S, Bhat SG. Thermostable bacteriocin BL8 from Bacillus licheniformis isolated from marine sediment. J App Microbiol. 2013;114:688-94.
Gray EJ, Lee KD, Souleimanov AM, Di Falco MR. et al. A novel bacteriocin, thuricin 17, produced by plant growth promoting Rhizobacteria strain Bacillus thuringiensis NEB17: Isolation and classification. J Appl Microbiol. 2006;100:545-554.
Skalka B. Bacteriocin typing of Staphylococci. Acta Veterniaria Brno. 1992; 61: 179-187.
Joel EL, Bhimba BV. A antibacterial activity and GCMS analysis of secondary metabolites produced by the mangrove plant Rhizophora mucronata against MRSA. [Quick Edit] Intal J Appl Bioengineering. 2010; 4: 25-28.
Patra JK, Thatoi,NH. Metabolic diversity and bioactivity screening of mangrove plants: a review. Acta Physiol Plant. 2011; 33:1051–1061.
Wangensteen H, Klarpås L, Alamgir M, Samuelsen,ABC, Malterud KE. Can scientific evidence support using Bangladeshi traditional medicinal plants in the treatment of diarrhoea? A Review on seven plants. Nutrients. 2013; 5: 1757-1800.