Validated HPTLC method for standardization of an ayurvedic powdered formulation-Panchsakar churna
Keywords:
Panchsakar Churna, HPTLC, Standardization, Sennoside, Gallic acidAbstract
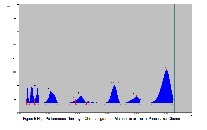
Objective: Panchsakar Churna is a marketed ayurvedic powdered formulation containing Cassia angustifolia and Terminalia chebula as the main ingredients. Present study aims to standardize Panchsakar Churna based upon chromatographic and spectral studies. Methods: This study developed an efficient and reliable high performance thin layer chromatographic method for quantification of Sennoside B and Gallic acid in Panchsakar Churna. The developed method was validated for linearity, accuracy, precision, sensitivity and purity. Results: Linearity of peak area was tested in the range 0.310-3.100µg/spot (r=0.9835) and 0.200-2.000 µg/spot (r=0.9929), Accuracy was assessed with recovery and the recoveries were 99.909-100.330 % and 99.690-100.562 % (n=3 for each sample), Precision were 0.257-2.580 and 0.425-2.201, sensitivity was determined with respect to limit of detection and limit of quantification, limit of detection were 73.7634 ng/spot and 50.1556 ng/spot, while limit of quantification were 245.8781ng/spot and 167.1853ng/spot, purity of the spot were 0.9984-0.9999 (r) and 0.9977-0.9998 (r) respectively for Sennoside B and Gallic acid. Conclusion: Sennoside B and Gallic acid can be used as possible marker compound for standardization of Panchsakar Churna.
References
Elamthuruthy AT, Shah CR, Khan TA, Tatke PA, Gabhe SY. Standardization of marketed Kumariasava an Ayurvedic Aloe vera product, J of Pharm and Biomed Anal. 2005; 37: 937–941.
Mukherjee PK. Quality control of Herbal Product, 1st ed. New Delhi, India, Buisness Horizons Ltd., 2002. p. 6-7.
Kumar P, Jha S, Standardization of an Ayurvedic powdered formulation by modified Lycopodium spore and Spectrophotometric method, Ind J Trad Knowledge. 2011; 10(4): 604-607.
Pathak RR. Ayurved Sarsangrah. 12th ed. Allahbad, India: Shree Baidyanath Ayurved Bhawan Ltd.; 2003. p. 589.
Kumar P, Jha S, Naved T. Pharmacognostical characterization of an Ayurvedic powdered formulation: Panchsakar Churna, Int J Res in Pharm and Chem. 2011; 1(4): 1034-1041.
Shao WS, Hsiu TS. Validated HPLC method for determination of sennosides A and B in senna tablets, J of Pharm and Biomed Anal. 2002; 29: 881–894.
Bhattacharjee SK. A handbook of medicinal plant, Jaipur, India, Pointer Publisher; 2004. p. 74-79.
Jeganathan NS, Kannan K. HPTLC method for estimation of Ellagic acid and Gallic acid in Triphala churnam formulations, Res J of Phytochem. 2008; 2(1): 1-9.
Mukherjee PK, Wahile A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicine, J of Ethnopharmacol. 2006; 103(1): 25-35.