Chemical constituent, antibacterial and antioxidant activity of crude extract and oil fraction of L. abyssinica
Keywords:
Ludwigia abyssinica, GC-MS, Column chromatography, antioxidant, antibacterialAbstract
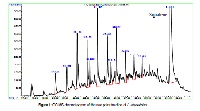
The antibacterial and antioxidant activities of CH3Cl/MeOH crude extract and a nonpolar fraction from the CHCl3 phase of Ludwigia abyssinica were investigated using broth dilution method and 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging assay respectively. Chemical composition was determined by gas chromatography coupled with mass spectrometry using Agilent Technologies 7890A GC system-Agilent Technologies 5975C inert XL EI/CI MSD. Bioassays show that L. abyssinica is active on the different test organisms with the MIC ranging from 0.5 to 1.5 mg/ml for the crude extract and 0.2 to 0.5 mg/ml for the non polar oil fraction. The oil extract of L. abyssinica exhibited a good antioxidant activity with radical scavenging activity (IC50) = 89.18 µg/ml which is about 1.5 times the antioxidant activity of ascorbic acid used as control, while that of crude extract was 413.74 µg/ml. From the gas chromatography coupled with mass spectrometry analyses, 12 hydrocarbons were identified with octadecane (5.67%), pentadecane (6.87%) and squalene (57.18%) the major components. These results offer a plat form of using L. abyssinica for alternative and complementary medicine.
References
Bando, N.; Hayashi, H.; Wakamatsu, S.; Inakuma, T.; Miyoshi, M.; Nagao, A.; Yamauchi, R.; Terao, J. Participation of singlet oxygen in ultraviolet-a-induced lipid peroxidation in mouse skin and its inhibition by dietary beta-carotene: an ex vivo study. Free Radic. Biol. Med. 2004, 37, 1854-1863.
Burkill HM (1997). The useful plants of West Tropical Africa. Royal Botanical Gardens. Kew, 14: 303-308.
Carbonnelle, B., Denis, F., Marmonier, A., Pinon, G., Vorgue, R., 1987. Techniques usuelles de Bactériologie Médicale. SIMEP Paris. p.330.
Carlson CF, Mee BJ, Riley TV: Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 2002, 46:1914-1920.
Chen CJ, Hoch PC, Raven PH. Systematics of Epilobium (Onagraceae) in China. Systematic Bot. Monogr 1992; 34:1-209
Das B, Kundu J, Bachar SC, Uddin MA, Kundu JK. Antitumor and antibacterial activity of ethyl acetate extract of Ludwigia hyssopifolia Linn and its active principle piperine. Pak. J. Pharmaceut. Sci 2007; 20(2): 128-131.
Gastsing, D.; Mbah, J.A.; Garba I. H.; and Tane, P., 2006. An antisalmonellal agent from the leaves of Glossocolyx buvipes Benth (Monimicaceae). Pakistan Journal of Biological Sciences, 9 (1). 84-87
Gerald N Teke, Paul K Lunga, Hippolyte K Wabo, Jules-Roger Kuiate, Gerard Vilarem, Geraldine Giacinti, Haruhisa Kikuchi, Yoshiteru Oshima. Antimicrobial and antioxidant properties of methanol extract, fractions and compounds from the stem bark of Entada abyssinica Stend ex A. Satabie. BMC Complementary and Alternative Medicine; 2011; (11:57) 1–8
Joyce, E.C., Rebeca, P.M., Lidiane, N.B., Luiz, C.D.S. and Ary, F.J., 2006. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Memorias Do Instituto Oswaldo Cruz, Rio de Janeiro, 101(4): 387-390
Kahl R, Kappus H (1993). Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebensm. Unters. Forsch.A.
Kilic T: Analysis of essential oil composition of Thymbra spicata var.spicata: antifungal, antibacterial and antimycobacterial activities. J Biosci 2006, 61:324-8.
Koroch A, Juliani R, Zygadlo A: Bioactivity of essential oils and their components. In Flavours and fragrances. Edited by: Berger R. Heidelberg: Springer-Verlag; 2007:87-115.
Mativandlela, S.P.N., Lall, N. and Meyer, J.J.M., 2006. Antibacterial, antifungal and antitubercular activity of Pelargonium reniforme (CURT) and Pelargonium sidoides (DC) (Geraniaceae) root extracts. South African Journal of Botany 72, 232–237.
Niki, E., and Noguchi, N. (2000) Evaluation of Antioxidant Capacity. What Capacity Is Being Measured by Which Method? IUBMB Life 50, 323–329.
Obeid HK, Allen MS, Bedgood DR, Prenzler PD, Robards K (2005). Investigation of Australian olive mill waste for recovery of biophenols. J. Agric. Food Chem., 53: 9911-9920.
Oyedeji O, Oziegbe M, Taiwo FO. Antibacterial, antifungal and phytochemical analysis of crude extracts from the leaves of Ludwigia abyssinica A. Rich. and Ludwigia decurrens Walter. Journal of Medicinal Plants Research 2010; 5(7): 1192-1199
Satchell AC, Saurajen A, Bell C, Barnetson RS: Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol 2002, 47:852-855.
Senthilkumar G, Madhanraj P., Panneerselvam A 2011. Studies on the Compounds and Its Antifungal Potentiality of Fungi Isolated From Paddy Field Soils of Jenbagapuram Village, Thanjavur District, and South India. Asian J. Pharm. Res. 2011; Vol. 1: Issue 1, Pg 19-21
Stahl E: Thin Layer Chromatography. Springer-Verlag New York, USA;, 2. 1969.
Takeo, O., Masato, K., Keiko, S., Rika, O., Junko, M., Hiroshi, I., Hiroyuki, K., Toshi, A., Toshifumi, A. and Shigeo, M., 2004. In Vitro and in vivo Antibacterial Activities of the Tricyclic Ketolide Te-802 and its Analogs. The Journal of Antibiotics 57, 518-527.
Trambetta D, Castelli F, Sarpietro MG, Venuti V, Christani M, Daniele C,Saija A, Mazzanti G, Bisignano G: Mechanism of antibacterial action of three monoterpenes. Antimicrob Agents Chemother 2005, 49:2474-2478.
Van Esch GJ (1986). Toxicology of tert-butylhydroquinone (TBHQ). Food Chem. Toxicol., 24: 1063-1065.
Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.H.; Wang, Z.Q.; Mahesh, B., Satish, S., 2008. Antimicrobial activity of some important medicinal plants against plant and human pathogens. World Journal of Agricultural Sciences 4 839-843.
Wolf D, Peter S. Oxidative stress in phagocytes “The Enemy Within” Microscopy Research and Technique 2002; 57:441-455.
Yala, D., Merad, A.S., Mohamedi, D. and Ouar, K.M.N.. 2001. Classification and mode of action of antibiotics. Maghreb Medicine 91: 5-12.
Yogeswari S., S. Ramalakshmi, R. Neelavathy, J. Muthumary 2012. Identification and Comparative Studies of Different Volatile Fractions from Monochaetia kansensis by GCMS. Global Journal of Pharmacology 6 (2): 65-71, 2012