Antioxidant potential of polar and non polar solvent extracts of Aphanamyxis polystachya in vitro
Keywords:
Antioxidant activity, Aphanamixis polystachya, Amoora rohituka, Free radicalsAbstract
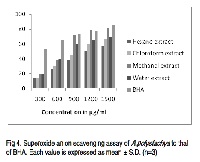
Aphanamixis polystachya (Wall.) Parker, vernacularly called “Amoora rohituka” is a medicinal plant belonging to the family of Meliaceae and appearing among Indian natural drugs used as remedy as an astringent and applied on swelling after a fall. In recent times anti carcinogenic ingredients are found from this plant. The search for safe therapeutic molecules suitable for long term use are most needed today to prevent the progression of free radical mediated diseases. Hence, the antioxidant activity of polar and non-polar solvent extracts of the leaf of Aphanamixis polystachya was evaluated in a series of in vitro assay involving free radicals and reactive oxygen species. The extract exhibited its scavenging effect in concentration dependent manner depending on the solvent extract on superoxide anion radicals, DPPH radical scavenging and property of metal chelating and reducing power. The extract has shown considerably good value for total reducing power, total phenolics, flavanoids and flavanol content.
References
Kirtikar, K. R., and B. D. Basu. 1980. Indian Medicinal Plants, 2nd ed. Dehradun, India, pp 551 – 553.
Rabi, T., D. Karunagaran, M. K. Nair, and V. N. Bhattathiri. 2002. Cytotoxic activity of amooranin and its derivatives. Phytother Res. 16(1):84 – 86.
Rabi, T., C. Ramachandran, H. B. Fonseca, R. P. Nair, A. Alamo, S. J. Melnick, et al., 2003. Novel drug amooranin induces apoptosis through caspase activity in human breast carcinoma cell lines. Breast cancer Res and Treat. 80:321 – 330.
Ramachandran, C., P. K. R. Nair, A. Alamo, C. B. Cochrane, E. Escalon, and S. J. Melnick 2006. Anticancer effects of amooranin in human colon carcinoma cell line in vitro and in nude mice xenografts. Int J. of Cancer. 119 (10):2443 – 2454.
Chan, L. L., S. George , T. Ahmad , S. L Gosangari , A. Abbasi A, and B. T. Cunningham. 2011. Cytotoxicity effects of Amoora rohituka and Chittagonga on breast and pancreatic cancer cells. Evidence-Based Complement and Altern Med. 2011, 8 pages, Article ID 860605.
Harmon, A. D., U. Weiss, and J. V. Silverton. 1979. The structure of rohitukine, the main alkaloid of Amoora rohituka (syn. Aphanamixis polystachya) (Meliaceae). Tetrahedron Lett. 8:721-724.
Srivastava, S. K., S. D. Srivastava, and S. Srivastava. 2003. New biologically active limonoids and flavonoids from Aphanamixis polystachya. Ind J. of Chem, Sec B: Org Chem. 42:3155 – 3158.
Chowdhury, R., C. M. Hasan, and M. A. Rashid. 2003. Guanine sesquiterpenes from Amoora rohituka. Phytochem. 62(8):1213 – 1216.
Sadhu, S. K., P. Phattanawasin, M. S. K. Choudhuri, T. Ohtsuki, M. Ishibashi. 2006. A new lignan from Aphanamixis polystachya. J. of Nat Med. 60, 258 – 260.
Krishnaraju, A. V., C. V. Rao, T. V. N. Rao, K. N. Reddy, and G. Trimutulu. 2009. In vitro and In vivo antioxidant activity of Aphanamixis polystachya bark. Amer. J. of Infect Dis. 5(2):60 – 67.
Sarla, S., M. A. Prakash, R. Apeksha, and C. Subhash. 2011. Free radical scavenging (DPPH) and Ferric Reducing Ability (FRAP) of Aphanamixis polystachya (Wall) Parker. Int J. of Drug dev and Res. 3(4):271 – 274.
Gheldof, N., and N. J. Engeseth. 2002. Antioxidant capacity of honeys from various floral sources based on the detection of oxygen radical absorbance capacity and inhibition of In vitro lipoprotein oxidation in human serum samples. J. of Agri and Food Chem. 50:3050 – 3055.
Sen, S., R. Chakraborthy, C. Sridhar, Y. S. R. Reddy, and Biplab De. 2010. Free radicals, antioxidants, diseases and phytomedicines: Current status and future prospect. Int J. of Pharma Sci Rev and Res. 3(1):91 – 100.
Goldfarb, A. H. 1993. Antioxidants: Role of supplementation to prevent exercise-induced oxidative stress. Med and Sci in Sports and Exer. 25:232 – 236.
Brand – Williams, W., M. E. Cuvelier , and C. Berset. 1995. Use of a free radical method to evaluate antioxidant activity. Food Sci and Technol. 28:25 – 30.
Sultanova, N., T. Makhmoor, Z. A. Abilov, Z. Parween, V. B. Omurkamzinova, Atta-ur-Rahman, and I. Choudhary. 2001. J. of Ethnopharmacol. 78:201 – 205.
Yen, G. C., and H. Y. Chen. 1995. Antioxidant activity of various tea extracts in relation to their antimutagenecity. J. of Agri and Food Chem. 43:27 – 32.
Dinis, T. C. P., V. M. C. Madeira and M. L. M. Almeida. 1994. Action of phenolic derivatives (acetoaminophen salicylate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch of Biochem and Biophys. 315:161 – 169.
Liu, F., V. E. C. Ooi and S. T. Change. 1997. Free radical scavenging of mushroom polysaccharide extracts. Life Sci. 60:763 – 771.
Sudha, G., M. Sangeetha Priya, R. Indhushree, and S. Vadivukkarasi. 2011. In vitro free radical scavenging activity of raw Pepino fruit (Solanum muricatum). Int J. of Curr Pharma Res. 3(2):137 – 140.
Miliauskas, G., P. R. Venskutonis, and T. A. Van Beek. 2004. Screening of radical scavenging activity of some medicinal and aromatic plants. Food Chem. 85:231 – 237.
Singleton, V. L., and J. A. Rossi. 1965. Colorimetry of total phenolics with phosphotungstic acid reagents. Amer J. of Enol and Viticult. 16:144 – 158.
Philips, A., S. Philips, V. Arul, B. Padmakeerthiga, V. Renju, S. Santha, et al. 2010. Free radical scavenging activity of leaf extracts of Indigofera aspalathoides – An in vitro analysis. J. of Pharma Sci Res. 2:322 – 328.
Rajamanikandan, S., T. Sindhu, D. Durgapriya, D. Sophia, P. Ragavendran, and V. K. Gopalakrishnan. 2011. Radical scavenging and Antioxidant activity of ethanolic extract of Mollugo nudicaulis by In vitro assays. Ind J. of Pharma Edu and Res. 45 (4):310 – 316.
Quang-Vinh Nguyen., and Jong-Bang Eun. 2011. Antioxidant activity of solvent extracts from Vietnamese medicinal plants. J. of Med Plants Res. 5(13):2798 – 2811.
Al-Mamun, M., K. Yamaki , T. Masumizu , Y. Nakai , K. Saito, H. Sano, et al. 2007. Superoxide anion radical scavenging activities of herbs and pastures in northern Japan determined using electron spin resonance spectroscopy. Int. J. of Biol Sci. 3:349 – 355.