Moringa oleifera Lam. leaves prevent Cyclophosphamide-induced micronucleus and DNA damage in mice
Keywords:
M. oleifera, Anti-genotoxi, Micronucleus assay, Comet assay, ChemopreventionAbstract
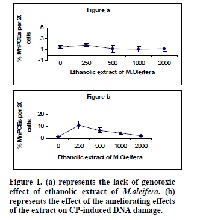
Chemoprotective effect of ethanolic extract of Moringa oleifera Lam leaves was evaluated on cyclophosphamide (CP)-induced genotoxicity in the mouse. Animals were pre-treated with the extract for seven consecutive days at doses of 250, 500, 1000 and 2000 mg/kg b.w. Micronucleus in bone marrow and comet (DNA damage) in the liver were performed. Cyclophosphamide was administered intra-peritoneally on day 7 and Mice were sacrificed after 24 hours. In CP treated animals, statistically significant induction of micronuclei in polychromatic erythrocytes (PCE) was recorded. However, in the animals pre-treated with the extract, the percentage of CP-induced MN decreased with increasing concentration of the extract. Results of comet assay showed similar decrease in DNA damage in mice pre-dosed with the extract. These results point out to the presence of chemopreventive phytoconstituents in the crude extract offering protection against CP-induced genotoxicity in the mouse.
References
Renner HW. In vivo effect of single or
combined dietary antimutagens on mutagen
induced chromosomal aberrations. Mutat
Res. 1990; 244: 185-188.
Hartwell JL. Plants used against cancer: a
survey. Lloydia. 1967-197; 130-34.
Fahey JW, Zalcmann AT and Talalay P. The
chemical diversity and distribution of
glucosinolates and isothiocyanates among
plants. Phytochemistry 2001; 56(1): 5-51.
[Corrigendum: Phytochemistry 59: 237].
Bennett RN, Mellon FA, Foidl N, Pratt JH,
DuPont MS, Perkins L and Kroon PA.
Profiling glucosinolates and phenolics in
vegetative and reproductive tissues of the
multi-purpose trees Moringa oleifera L.
(Horseradish tree) and Moringa stenopetala L.
J. Agri. Food Chem.2003; 3546-3553.
Fahey JW, Dinkova-Kostova AT and Talalay
P. The “Prochaska” microtiter plate bioassay
for inducers of NQO1. Chapter 14 in Methods
in Enzymology, 2004. Vol. 382, Part B, pp.
-258 (Eds.) H. Sies & L. Packer, Elsevier
Science, San Diego, CA.
Guevara AP, Vargas C, Sakurai H, Fujiwara
Y, Hashimoto K, Maoka T, Kozuka M, Ito
Y, Tokuda H and Nishino H. An antitumor
promoter from Moringa oleifera Lam. Mutat.
Res.1999; 440: 181-188.
Murakami A, Kitazono Y, Jiwajinda S,
Koshimizu K, Ohigashi H. Niaziminin,
thiocarbamate from the leaves of Moringa
oleifera, holds a strict structural requirement
for inhibition of tumor-promoter-induced
Epstein–Barr virus activation. Planta Med.
; 64 (4): 319–323.
Bharali R, Tabassum J and Azad MRH.
Chemomodulatory effect of Moringa oleifera,
Lam, on hepatic carcinogen metabolizing
enzymes, antioxidant parameters and skin
papillomagenesis in mice. Asian Pacific
Journal of Cancer Prevention 2003; 4: 131-
Kirtikar KR and Basu BD. In: Bishen Singh
and Mahendra Pal Singh (Eds) Indian
Medicinal Plant, Dehradun, 1935. pp. 677–
Caceres A, Saravia A, Rizzo S, Zabala L,
Leon ED, Nave F. Pharmacological properties
of Moringa oleifera : screening for
antispasmodic, anti-inflammatory and diuretic
activity. J. Ethnopharmacol. 1992; 36, 233–
Udupa SL, Udupa AL, Kulkarni DR. Studies
on the anti-in.ammatory and wound healing
properties of Moringa oleifera and Aegle
marmelos. Fitoterapia 1994; 65: 119–123.
Pal SK, Mukherjee PK, Saha BP. Studies on
the antiulcer activity of Moringa oleifera leaf
extract on gastric ulcer models in rats.
Phytother Res.1995; 9: 463–465.
Pal SK, Mukherjee PK, Saha K, Pal M and
Saha BP. Studies on some
psychopharmacological actions of Moringa
oleifera Lam. (moringaceae) leaf extract.
Phytother Res 1996; 10: 402–405.
Jaiswal, Prashant KR, Amit K, Shikha M and
Geeta W. J. Ethnopharmacol. 2009; 123:392–
Shukla S, Mathur R and Prakash AO. Effects
of aqueous extract of Moringa oleifera Lam.
on the periodicity of oestrous cycle in adult
intact rats. Indian J Pharma sci. 1981; 49:
–219.
Prakash A. Ovarian response to aqueous
extract of Moringa oleifera. Fitoterapia
;59: 89–91.
Tahiliani P and Kar A. Role of Moringa
oleifera leaf extract in regulation of thyroid
hormone status in adult male and female rats.
Pharma. Res. 2000; 41: 319–323.
Makkar HPS and Becker K. Nutritional value
and anti-nutritional components of whole and
ethanol extracted Moringa oleifera leaves.
Animal Feed Sci Tech 1996; 63: 211–228.
Freiberger CE, Vanderjagt DJ, Pastuszyn A,
Glew RS, Mounkaila G, Millson M and Glew
RH. Nutrient contents of the edible leaves of
seven wild plants from Niger. Plant Foods for
Human Nutrition 1998; 53, 57–69.
Nambiar VS and Seshadri S. Bioavailability
trials of ¡-carotene from fresh and dehydrated
drumstick leaves (Moringa oleifera) in a rat
model. Plant Foods for Human Nutrition.
; 56: 83–95.
Lakshminarayana R, Raju M, Krishnakantha
TP and Baskaran V. Determination of major
carotenoids in a few Indian leafy vegetables
by high-performance liquid chromatography.
J Agri Food Chem 2005; 53, 2838– 2842.
Sanchez MDI, Lopez CJ and Vazquez NJR.
High-performance liquid chromatography
method to measure ²- and ¡-tocopherol in
leaves, flowers and fresh beans from Moringa
oleifera. J. Chrom. 2006; 1105: 111–114.
Mondal S, Chakraborty I, Pramanik M, Rout
D and Islamm SS. Structural studies of an
immunoenhancing polysaccharide isolated
from mature pods (fruits) of Moringa oleifera
(Sajina). Med Chem Res 2004; 13: 390–400.
Nair AGR and Subramanian SS. Pigments of
the lowers of Moringa pterygosperma. Curr
Sci. 1962; 31: 155–156.
Siddhuraju P and Becker K. Antioxidant
properties of various solvent extracts of total
phenolic constituents from three different
agro climatic origins of drumsticks tree
(Moringa oleifera Lam.) leaves. J. Agri. Food
Chem. 2003; 51: 44-55.
Chuang PH, Lee CW, Chou JY, Murugan M,
Shieh BJ and Chen HM. Antifungal activity
of crude extracts and essential oils of
Moringa oleifera Lam. Biores. Tech. 2007;
, 232–236.
Nepolean P, Anitha J and Emilin RR.
Isolation, analysis and identification of
phytochemicals of antimicrobial activity of
Moringa oleifera Lam. Current Biotica. 2009;
(1).
Rao AV, Devi PU and Kamath R. In vivo
radioprotective effect of Moringa oleifera
leaves. Indian J Exp Biol. 2001; 39(9): 858-
Krishna G and Hayashi M. In vivo rodent
Micronucleus assay: protocol, conduct and
data interpretation. Mutat Res. 2000; 455:
-166.
Sasaki YF, Tsuda S, Izumiyama F, Nishidate
E. Detection chemically induced DNA lesions
in multiple mouse organs (liver, lung, spleen,
kidney, and bone marrow) using the alkaline
single cell gel electrophoresis (Comet) assay.
Mutat Res 1997; 388: 33– 44.
Regildo MGS, Neila CS, Ulrich G, Mário AS.
Antigenotoxic effects of Mandevilla velutina
(Gentianales, Apocynaceae) crude extract on
cyclophosphamide-induced micronuclei in
Swiss mice and urethane-induced somatic
mutation and recombination in Drosophila
melanogaster. Gen. Mol. Bio .2008; 31(3).
Schmid W. The micronucleus test. Mutat Res.
; 31:9-15.
Singh NP, McCoy MT, Tice RR and
Schneider EL. A simple technique for
quantitation of low levels of DNA damage in
individual cells. Exp Cell Res 1988; 175:184
–191.
Collins AR, Duthie SJ and Dobson VL.
Direct enzymic detection of endogenous
oxidative base damage in human lymphocyte
DNA. Carcinogenesis 1993; 14, 1733–1735.
Kanekal S, Fraiser L and Kehrer JP.
Pharmacokinetics, metabolic activation and
lung toxicity of cyclophosphamide in
C57BL6 and ICR mice. Tox Appl Pharmacol.
; 114:1-8.
Chumark P, Khunawat P, Sanvarinda Y,
Phornchirasilp S, Morales NP. The in vitro
and ex vivo antioxidant properties,
hypolipidaemic and antiatherosclerotic
activities of water extract of Moringa oleifera
Lam Leaves. J Ethnopharmacol. 2008; 116,
–446.
Iqbal S and Bhanger MI. Effect of season and
production location on antioxidant activity of
Moringa oleifera leaves grown in Pakistan. J
Food Comp Anal. 2006; 19: 544–551.
Dahot MU. Antimicrobial activity of small
protein of Moringa oleifera leaves. Journal of
the Islamic Academy of Sciences 1998; 11(1):
pp.
Costa-Lotufo LV, Khan MTH, Ather A,
Wilke DV, Jimenez PC, Pessoa C, de Moraes
MEA and de Moraes MO. Studies of the
anticancer potential of plants used in
Bangladeshi folk medicine. J
Ethnopharmacol. 2005; 99: 21-30.
Gilani AH, Aftab K, Suria A, Siddiqui S,
Saleem R, Siddiqui BS and Faizi S..
Pharmacological studies on hypotensive and
spasmolytic activities of pure compounds
from Moringa oleifera. Phyto res 1994; 8(2):
-91.
Greenwald P, Clifford CK and Miner JA.
Diet and Cancer prevention. Eur J
Cancer.2001; 37, 948- 965.
Steinmetz KA and Potter JD. Vegetables,
fruit, and cancer. II. Mechanisms, Cancer
Causes Control, 1991; 2, 427-442.
Steinrnetz KA and Potter JD.. Vegetables,
fruit, and cancer. I. Epidemiology, Cancer
Causes Control, 1991; 2, 325-357.
Chorvatoviovfi D and Bauer V. Stobadineinhibitor of cyclophosphamide-induced
micronuclei in mice. Mutagenesis 1994; 9,
-244.