Screening for the antimicrobial activity of Salvadora persica extracts against Enterococcus faecalis and Candida albicans
Keywords:
Antimicrobial, C.albicans, E. faecalis, S.persicaAbstract
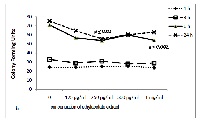
Antimicrobial resistance of Enterococcus faecalis (E. faecalis) and Candida albicans (C.albicans), frequently implicated in dental infections, remains a challenge. Using a variety of solvents this study was performed to screen the antimicrobial activity of Salvadora persica (S. persica) extracts against these two organisms. Seven extracts of S. Persica were prepared using hexane, chloroform, ethyl acetate, methanol-soluble, methanol-insoluble, ethanol, and water. Antimicrobial activities against E. faecalis and C.albicans were assessed by colony forming unit (CFU) counts after 1, 3, 6 and 24 hours of exposure using doubling dilutions of the extracts ranging between 125 µg to 1mg/ml. Among the extracts of S. persica tested, hexane extract induced a steady and progressive reduction in CFUs of both the E. faecalis (p<0.001) and C. albicans (p<0.01) at all concentrations beginning after 3 hours until 24 hours. Progressive inhibition of E. faecalis CFUs was also observed for ethanol beginning at 3 hours until 24 hours (p <0.001) and chloroform only at 24 hours (p<0.001) at all concentrations. Ethyl acetate extract of S. persica was effective against C. albicans at 250µg/mg after 6 hours (p<0.02) and 24 hours (p <0.002). No significant changes were observed in any of the other tested extracts of S. persica. Hexane extract of S. persica was found to exhibit maximum antimicrobial activity against E. faecalis and C.albicans. Further studies are recommended for evaluation of this extract as an effective anti-microbial agent.
References
Salehi P, Momeni Danaie S. Comparison of the antibacterial effects of persica mouthwash with chlorhexidine on streptococcus mutans in orthodontic patients. DARU. 2006; 14 (4): 178-182.
Almas K. The effect of Salvadora persica extract (miswak) and chlorhexidine gluconate on human dentin: a SEM study. J Contemp Dent Pract. 2002; 3(3): 27-35.
Almas K, Skaug N, Ahmad I. An in vitro antimicrobial comparison of miswak extract with commercially available non-alcohol mouthrinses. Int J Dent Hyg. 2005; 3(1): 18-24.
Koch S, Hufnagel M, Theilacker C, Huebner J. Enterococcal infections: host response, therapeutic, and prophylactic possibilities. Vaccine. 2004; 22 (7) :822–830.
Facklam RR, Carvalho MS, Teixeira LM. History, taxonomy, biochemical characteristics, and antibiotic susceptibility testing of enterococci. In: Gilmore MS, (eds) The Enterococci: pathogenesis, molecular biology and antibiotic resistance. Washington, DC: Am Soc Microbiol; 2002. p. 1–54.
Bystrom A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985; 18(1): 35–40.
Sundqvist G. Associations between microbial species in dental root canal infections. Oral Microbiol Immunol. 1992; 7 (5): 257–262.
Sundqvist G, Figdor D, Persson S, Sjogren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.1998; 85 (1): 86–93.
Sedgley C, Nagel A, Dahlen G, Reit C, Molander A. Real-time quantitative polymerase chain reaction and culture analyses of Enterococcus faecalis in root canals. J Endod. 2006; 32 (3): 173-177.
Siqueira JF, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J.1999; 32 (5): 361–369.
Kayaoglu G, Orstavik D. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Crit Rev Oral Biol Med.2004;15 (5): 308-320.
Samaranayake LP, Yaacob H. Classification of oral candidosis. In: Samaranayake LP, MacFarlane TW (eds) Oral Candidosis. London, UK: Butterworth; 1990. P. 156–83.
Egan MW, Spratt DA, Ng YL, Lam JM, Moles DR, Gulabivala K .Prevalence of yeasts in saliva and root canals of teeth associated with apical periodontitis. Int Endod J. 2002; 35(4): 321-329.
Odds FC. Candida and Candidosis: A Review and Bibliography, 2nd edn; London: Bailliere Tindall: WB Saunders;1988.
Al lafi T, Ababneh H. The effect of the extract of the miswak (chewing sticks) used in Jordan and the Middle East on oral bacteria. Int Dent J.1995; 45(3): 218-222.
Al-Bagieh NH, Idowu A, Salako NO. Effect of aqueous extract of miswak on the in vitro growth of Candida albicans. Microbios. 1994; 80 (323): 107-113.
Singh R, Dar SA, Sharma P. Antibacterial activity and toxicological evaluation of semi purified hexane extract of Urticadioica leaves. Res J Med Plant. 2012; 6(2): 123-135.
Elzaawely AA, Xuan TD, Tawata S . Antioxidant and antibacterial activities of Rumex japonicus HOUTT. Aerial parts. Biol Pharm Bull. 2005; 28(12): 2225-2230.
Uma B and Parvathavarthini R. Antibacterial effect of hexane extract of sea urchin, Temnopleurus alexandri (Bell, 1884). Int J Pharm Tech Res. 2010; 2(3): 1677-1680.
Johann S, P SA N, Lima LARS, Cisalpino PS, Cota B, Alves TMA, Siqueira EP, Zani CL. Antifungal activity of schinol and a new biphenyl compound isolated from Schinus terebinthifolius against the pathogenic fungus Paracoccidioides brasiliensis. Ann Clin Microbiol Antimicrob.2010; 9: 30.
Ghannoum MA, Rice LB. Antifungal agents: Mode of action, mechanisms of resistance and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999; 12(4): 501-517.
Julián E, Cama M, Martínez P, Luquin M. An ELISA for five glycolipids from the cell wall of Mycobacterium tuberculosis: Tween 20 interference in the assay. J Immunol Methods. 2001; 1; 251(1-2): 21-30.
Beevi SS, Mangamoori LN, Dhand V, Ramakrishna DS. Isothiocyanate profile and selective antibacterial activity of root, stem, and leaf extracts derived from Raphanus sativus L. Foodborne Pathog Dis. 2009; 6 (1): 129-136.
Al-Bagieh NH, Weinberg ED. Benzylisothiocyanate: a possible agent for controlling dental caries. Microbios. 1988; 39:143-151.
Sofrata A, Santangelo EM, Azeem M, Borg-Karlson AK, Gustafsson A, Pütsep K . Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against Gram-negative bacteria. PLoS One. 2011; 6 (8):e23045.
Abo Al- Samh DA, Al-Bagieh NH. A study of the antimicrobial activity of the miswak ethanolic extract in vitro. Biomed Letters. 1996; 53: 225-38.
AbdElRahman HF, Skaug N, Francis GW. In vitro antimicrobial effects of crude miswak extracts on oral pathogens. Saudi Dent J. 2002; 14 (1): 26-32.
Al-Sohaibani S, Murugan K. Anti-biofilm activity of Salvadora persica on cariogenic isolates of Streptococcus mutans: in vitro and molecular docking studies. Biofouling. 2012; 28 (1): 29-38.
Guedes EA, Dos Santos Araujo MA, Souza AK, de Souza LI, de Barros LD, de Albuquerque Maranhao FC, Sant,ana AE .Antifungal activities of different extracts of Marine Macroalhae against dermatophytes and candida species. Mycopathologia. 2012; 174 (3): 223-232
Noumi E, Snoussi M, Hajlaoui H, Valentin E, Bakhrouf A . Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur J Clin Microbiol Infect Dis. 2010; 29 (1): 81-88.
Almas K. The antimicrobial effects of seven different types of Asian chewing sticks. Odontostomatol Trop. 2001; 24(96): 17-20.
Paliwal S, Chauhan R, Siddiqui A, Paliwal S, Sharma J . Evaluation of antifungal activity of Salvadora persica Linn. leaves. Nat Prod Radiance.2007; 6 (5): 372-374.
Al-Mohaya MA, Darwazeh A, Al-Khudair W. Oral fungal colonization and oral candidiasis in renal transplant patients: the relationship to Miswak use. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.2002;93 (4): 455-460.
Al-Bayati FA, Sulaiman KD. In vitro antimicrobial activity of Salvadora persica L. extracts against some isolated oral pathogens in Iraq. Turk J Biol. 2008;32: 57-62.
Sher H, Al-yemeni MN, Wijaya L . Ethnobotanical and antibacterial potential of Salvadora persica l: A well known medicinal plant in Arab and Unani system of medicine. J of Med Plants Res.2011; 5(7): 1224-1229.