Antibacterial activity and Phytochemical analysis of Cardanthera difformis Druce leaf extracts from West Bengal, India.
Keywords:
Agar well diffusion method, Antibacterial activity, Cardanthera difformis, PhytochemicalsAbstract
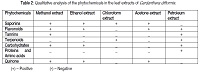
In rural and backward areas of west Bengal of India several plants are commonly used as herbal medicine for the treatment of infectious diseases without any phytochemical and biological information in detail. The current study was to investigate the antibacterial activity and phytochemical analysis of the leaf extracts of Cardanthera difformis. Methanol, ethanol, chloroform, acetone and petroleum ether extracts of shed dried plant leaves of Cardanthera difformis were tested for antibacterial activity and phytochemical screening.The antibacterial behaviour of different extracts (methanol, acetone, ethanol, chloroform and petroleum ether) of leaves of Cardanthera difformis using the standard well diffusion assay were investigated against the seven strains of bacterial species, viz., Enterobactor faecalis, Eschirichia coli, Klebsiella pneumonia, Pseudomonous aerusinosa, Salmonella typhi, Shiegella dysentriae and Staphylococcus aureus.. Among the various solvent extract the acetone leaf extract exhibit the best antibacterial property followed by chloroform, ethanol, methanol and petroleum ether. The ethanol extract showed significant activity against Staphylococcus aureus, Pseudomonous aeruginosa, Enterobactor faecalis and Shiegella dysentriae. The acetone extract showed the highest magnitude of inhibition against Salmonella typhi among the extracts under study. Phytochemical screening of the leaf extracts using standard qualitative methods revealed the presence of saponins, flavonoids, terpenoids, tannins, quinone, carbohydrates and amino acids. Both the petroleum ether and methanol extracts showed the maximum efficiency for biochemical constituents. The present study experimentally proved the justification of traditional use of C.difformis for the treatment of various diseases and reported for the first time in the world to focus the antibacterial activity of leaf extracts of C.difformis and may lead to the development of a new generation of drugs having both chemotherapeutic and chemo preventive properties in future.
References
Kala CP, Dhyani PP, Sajwan BS. Developing the medicinal plants sector in Northern India: challenges and opportunities. J Ethanobiol Ethnomed. 2006;2:32.
Pei SJ. Ethnobotanical approaches of traditional medicinal studies: some experiences from Asia. Pharm Biol.2001;39:74-79.
Das DC, Sinha NK, Chattopadhyay JC, Das M, Samanta P. The use of Medicinal Plants for the treatment of Gonorrhoea and Syphilis in South West Bengal of India. Int J Phytomed. 2013;5(1).
Goossens H, Ferench M, Vander SR, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579-587.
Kinghorn AD, Chai HB, Sung CK, Keller WJ. The classical drug discovery approach to define bioactive constituents of botanicals. Fitoterapia.2011;82(1):71-79.
Cos P, Vlietinck AJ, BergheDV, Maes L. Anti-infective potential of natural products;how to develop a stronger in vitro ‘proof-of concept’. J ethnopharmacol.2006;106:290-302
Houghton PJ. Old yet new- pharmaceuticals from plants. J Chem Edu . 2001;78:175
Nahrstedt A, Butterweck V. Lessons learned from herbal medicinal products:the example of St John’s Wort. J Nat Prod. 2010;73:1015-1021
Gerald B. Biologically active compounds from marine organism. Phytother Res. 2001;15:89-94.
Newman DJ, Cragg GM.Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461-477.
Dossey A. Insects and there chemicals weaponry:new potential for drug discovery. Nat Prod Rep. 2010;27:1737-1757.
Dorman HJ, Deans SG. Antimicrobial agents from plants : activity of plant volatile oils. J Appl Microbiol. 2000;88:308-316.
Asghari J, Ondruschka B, Mazaheritehrani M. Extraction of bioactive chemical compounds from the medicinal Asian plants by microwave irradiation. J Med Plant Res. 2011;5:495-506.
Zaika LL. Species and herbs their antimicrobial activity and its determination. J.Food Safty. 1975;9:97-118.
Gordon MC, David J. Natural Product drug discovery in the next millenium. Pharm Bio. 2001;139: 8-17.
Vedavaty S, Mrudal V, Sudhakar A. Tribal medicine in Chitton district, Andhra Pradesh, India. Vedamse Books P Ltd. 1997.
Chan-Bacab MJ, Pena-Rodriguez LM. Plant natural products with leishmanicidal activity. Nat Prod Rep. 2001; 18 : 674 – 688.
Evans WC. Trease and Evan’s Pharmacognosy. 14th Edition. W B Saunders Co. 1996; 290.
Perez C, Paul M , Bazerque P. Antibiotic assay by agar by agar – well diffusion method. Acta Biol Med Exp. 1990 15 :113 -115.
Olurinola PF. A Laboratory Manual of Pharmaceutical Microbiology. National Institute for Pharmaceutical Research and Development, Abuja, Nigeria. 1996; 69-105.
Samanta K, Hossain E, Pal DK. Anthelmentic activity of Hygrophila difformis Blume. J Buffalo science. 2012; 1:35-38.
Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 14 edition. Nirali Prakasanan, Pune, India. 2008; 1 – 635.
Harborne JB, Harborne JB. Phytochemical methods ; A Guide to Modern Techniques of plant analysis. Champman and Hall, New York. 1998 ; 1- 320.
Harborne JB. Phytochemical methods : A Guide to Modern Techniques of plant of analysis. 3rd Edition. Chapman and Hall , New York. 1998; 1 – 302.
Rios JL, Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 2005;100:80-84.
Cao Y, Wei X, Xu H, Tang W. Antifungal properties of methanol extracts and its active compounds from Brickellia rosmarinifolia .Vent Fitoterapia. 2010;81(8):1176-1179.
Sule A, Ahmed QU, Samah OA, Omar MN, Hassan NM, Kamal LZM. Bioassay guided isolation of antibacterial compounds from Andrographis paniculata. Am J Appl Sci. 2011;8:525-534.
Tsuchiya H, Sato M, Miyazaki T, Fujuwara S, Tanigaki S, Ohayama M, Tanaka T, Linuma M. Comparative study on the antibacterial activities of phytochemical flavonones against methicillin resistant Staphylococcus aureus. J Ethnopharmacol. 1996;50:27-34.
Ya C, Gaffney SH, Lilley TH, Haslam E. Carbohydrate-Polyphenol Complexation.In: Chemistry and Significance of condensed tannins. Hemingway RW, Karchesy JJ, ed. In Plenum Press, New York.1988;553.
Nabila B, Fawzia BA, Tatjana PK. Antioxidant and Antimicrobial activities of Pistacia lentiscus and Pistacia atlantica extracts. Afr J Pharm Pharmacol.2008;2 (2):22-28.
Rupasinghe HP, Jackson CJ, Poysa V, Berardo CD, Bewley JD, Jenkinson J. Soyasapogenol A and B distribution in soyabean (Glycine max L. Merr) in relation to seed physiology, genetic variability and growing location. J Agr Food Chem.2003;51:5888-5894.
Argal A, Pathak AK. CNS activity of Calotropis gigantea roots. J Ethnopharmacol. 2006;106:142-145.
Malairajan P, Gopalakrishnan G, Narasimhan S, Veni KJK. Analgesic activity of some Indian medicinal plants. J Ethnopharmacol. 2006;106:425-8.