Immunomodulation potential of Artemisia capillaris extract in rat splenocytes
Keywords:
Artemisia capillaris, immunomodulation, cyclooxygenase-2, inducible nitric oxide synthase, cytokineAbstract
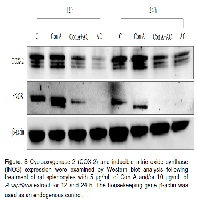
The extract of Artemisia capillaris has been used as a traditional medicine for hepatitis or bilious disorder, and some recent studies have revealed its antimicrobial, antioxidant, antiobesity, anticarcinogenic, antioxidant, and anti-inflammatory potential. The current study was designed to evaluate the potential immunomodulatory effects of A. capillaris methanol extract on quiescent- and concanavalin A (Con A)-stimulated rat splenocytes. Proliferation of splenocytes was enhanced in response to the Con A mitogen but decreased significantly following A. capillaris extract treatment compared to that in the control and the Con A only treatment. A. capillaris extract at 10 µg/mL significantly reduced Con A-induced proliferation, and the expression of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and proinflammatory cytokines as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). Another proinflammatory cytokine, interferon-gamma (IFN-γ), was not significantly altered by either Con A or A. capillaris extract treatments when compared with the control. iNOS expression was largely blocked, whereas COX-2 expression was significantly down-regulated by A. capillaris extract treatment. This study was carried out to investigate the potential immunomodulatory activities of A. capillaris extract in a Con A induced immune condition to explore its potential pharmacological activity.
References
Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bull World Health Organ. 1985; 63(6):965-981.
Kim JH, Kim DK, Kim JH. Genetic variation and relationship of Artemisia capillaris Thunb.(Compositae) by RAPD analysis. Korean J Plant Res. 2009; 22(3):242-247.
Chang HM, But PPH. Pharmacology and Applications of Chinese Material. Media, World Scientific, Singapore. 1987; 867–871.
Kim YS, Bahn KN, Hah CK, Gang HI, Ha YL. Inhibition of 7,12- dimethylbenz[a]anthracene induced mouse skin carcinogenesis by Artemisia capillaris. J Food Sci. 2008; 73:T16–20.
Lee J, Chae K, Ha J, Park BY, Lee HS, Jeong S, Kim MY, Yoon, M. Regulation of Obesity and lipid disorders by herbal extracts from Morusalba, Melissaofficinalis, and Artemisia capillaris inhigh-fat diet-induced obese mice. J Ethnopharmacol. 2008; 115: 263–270.
Luo H, Lin S, Ren F, Wu L, Chen L, Sun Y. Antioxidant and antimicrobial capacity of Chinese medicinal herb extracts in raw sheep meat. J Food Prot. 2007; 70:1440–1445.
Cho YH, Chiang MH. Research papers: Essential Oil Composition and Antibacterial Activity of Artemisia capillaries, Artemisia argyi, and Artemisia princeps. Kor J Intl Agri. 2001; 13(4):313-320.
Song, KW. 1996. Phytochemical study of polysaccaride fraction from Artemisia species. Sungkyunkwan Univ. Master thesis.
Wu TS, Tsang ZJ, Wu PL, Lin FW, Li CY, Teng CM, Lee KH. New constituents and antiplatelet aggregation and anti-HIV principles of Artemisia capillaris. Bioorg Med Chem. 2001; 9:77-83.
Malich G, Markovic B, Winder C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997; 124:179 – 192.
Choi JH, Kim DW, Yun N, Choi JS, Islam MN, Kim YS. Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J Nat Prod. 2011; 74:1055–1060
Jang SI, Kim YJ, Kim HJ, Lee JC, Kim HY, Kim YC. Scoparone inhibits PMA-induced IL-8 and MCP-1 production through suppression of NF-kappaB activation in U937 cells. Life Sci. 2006; 78:2937–2943.
Mase A, Makino B, Tsuchiya N, Yamamoto M, Kase Y, Takeda S. Active ingredients of traditional Japanese (kampo) medicine, inchinkoto, in murine concanavalin A-induced hepatitis. J Ethnopharmacol. 2010; 127:742–749.
Koo HN, Hong SH, Jeong HJ, Lee EH, Kim NG, Choi SD. Inhibitory effect of Artemisia capillaris on ethanol-induced cytokines (TNF-alpha, IL-1alpha) secretion in Hep G2 cells. Immunopharmacol Immunotoxicol. 2002; 24:441–453.
Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006; 63:321-329.
Esper RJ, Nordaby RA, Vilari ño JO, Paragano A, Cacharrón JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006; 5:4.
Trautwein C, Rakemann T, Malek NP, Plumpe J, Tiegs G, Manns MP. Concanavalin A-induced liver injury triggers hepatocyte proliferation. J Clin Invest. 1998; 101:1960–1969.
Klein C, Wüstefeld T, Heinrich PC, Streetz KL, Manns MP, Trautwein C. ME3738 protects from concanavalin A-induced liver failure via an IL-6-dependent mechanism. Eur J Immunol. 2003; 33:2251–226.
Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med 1996; 334:1717–1725.
Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor super-
families: integrating mammalian biology. Cell 2001; 104:487–501.
Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007; 96:41–101.
Koizuka S, Saito S, Obata H, Sasaki M, Nishikawa K, Takahashi K, Saito Y, Goto F. Oral etodolac, a COX-2 inhibitor, reduces postoperative pain immediately after fast-track cardiac surgery. J Anesth. 2004; 18(1):9-13.
Sass G, Heinlein S, Agli A, Bang R, Schümann J, Tiegs G. Cytokine expression in three mouse models of experimental hepatitis. Cytokine 2002; 19:115–20.
Jaruga B, Hong F, Kim WH, Gao B. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am J Physiol Gastrointest Liver Physiol. 2004; 287:G1044–52.
Fujikura S, Mizuhara H, Miyazawa Y, Fujiwara H, Kaneda K. Kinetics and localization of lymphoblasts that proliferate in the mutine liver after Concanavalin A administration. Biomed Res. 1996; 17:129–39.
Sass G, Koerber K, Bang R, Guehring H, Tiegs G. Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest. 2001; 107:439–47.
Ito Y, Abril Edward R, Bethea Nancy W, McCuskey Robert S. Role of nitric oxide in hepatic microvascular injury elicited by acetaminophen in mice. Am J Physiol Gastrointest Liver Physiol. 2004; 286:G60–7.
Tsuruoka N, Abe K, Wake K, Takata M, Hatta A, Sato T, Inoue H. Hepatic protection by glycyrrhizin and inhibition of iNOS expression in concanavalin A-induced liver injury in mice. Inflamm Res. 2009; 58(9):593-9.