Inhibitory effect of Dolichos biflorus extract on allergic airway inflammation and hyperresponsiveness in animal model of ovalbumin-induced asthma
Keywords:
Ovalbumin, BAL, Hyperresponsivenss, Dolichos biflorusAbstract
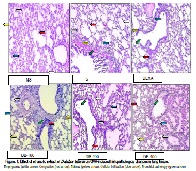
The context: Bronchial asthma is an allergic disorder characterized by airway hyper- responsiveness, infiltration of various inflammatory mediators and remodeling of the airways. Asthma is triggered by various factors like drugs, respiratory infection, dust, cold air, exercise, emotions, occupational stimuli, chemicals, histamine, and ovalbumin. Traditional medicinal plants are commonly used to treat or prevent asthma and its causes. Purpose of the study: Traditionally seeds of Dolichos biflorus are used in the treatment of cough, edema and asthma. The present study was designed to evaluate inhibitory effect of Dolichos biflorus extract on allergic airway inflammation and hyperresponsiveness in animal model of ovalbumin-induced asthma. Main findings: With the treatment of ethanolic extract of Dolichos biflorus (DB) seeds, there was significant decrease in inflammatory cell count, level of nitric oxide and total protein in bronchoalveolar lavage (BAL) fluid at the oral dose of 100, 200 and 400 mg/kg. DB also restored the level of lung antioxidant enzymes (LPO, GSH, SOD, Catalase) and reduced the wet/dry weight ratio. In histopathological examination of lung tissue, DB protected the lungs from pathological changes induced by OVA. Summary: These results indicate that ethanolic extract of Dolichos biflorus seeds decreased allergic airway inflammation and hyperresponsiveness by decreasing the infiltration of inflammatory cells in the airway. Potential implications: Our data suggests usefulness of Dolichos biflorus in prophylaxis and management of asthma.
References
Park S, Shin W, Seo J, Kim E. Anthocyanins inhibit airway inflammation and hyperresponsiveness in a murine asthma model. Food and Chemical Toxicology. 2007; 45: 1459–1467.
Patel PK, Patel KV , Gandhi TR. Evaluation of Effect of Taxus baccata Leaves Extract on Bronchoconstriction and Bronchial Hyperreactivity in Experimental Animals. Global Journal of Pharmacology. 2009; 3 (3): 141-148.
Clement YN , Williams AF, Aranda D, Chase R, Watson N, Mohammed R, Stubbs O, Williamson D. Medicinal herb use among asthmatic patients attending a specialty care facility in Trinidad. BMC Complementary and Alternative Medicine 2005, 5:3, pp 1-8.
Kirtikar KR, Basu BD. Indian Medicinal Plants. International Book Distributors, Dehradun. 3, 2005; 804-806.
Hazra B, Sarkar R, Mandal S, Biswas S, Mandal N. Studies on antioxidant and antiradical activities of Dolichos biflorus seed extract. African Journal of Biotechnology. 2009; 8(16): 3927-33.
Nanta R, Kale RK. Chemomodulatory effect of Dolichos biflorus Linn. on skin and forestomach papillomagenesis in Swiss albino mice. Indian J Exp Biol. 2011; 49: 483-90.
Garimella TS, Jolly CI, Narayanan S. In vitro studies on antilithiatic activity of seeds of Dolichos biflorus Linn. and rhizomes of Bergenia ligulata Wall. Phytother. Res. 2001; 15: 351-355.
Laskar S, Bhattarcharyya UK, Sinhababu A, Basak BK.Antihepatotoxic activity of kulthi (Dolichos biflorus) seed in rats. Fitoterapia. 1998; 69: 401-402.
Muthu AK, Sethupathy S, Manavalan R, Karar PK. Antioxidant potential of methanolic extract of Dolichos biflorus Linn. in high fat diet fed rabbits. Indian J. Pharmacol. 2006; 38: 131-132.
Khandelwal KR. Practical Pharmacognosy, Techniques and experiments. 2nd edition, Nirali prakashan, Pune. 2004; 149-153.
Chapman RW, Howard AH, Richard J, Celly C. Effect of inhaled roflumilast on the prevention and resolution of allergen-induced late phase airflow obstruction in Brown Norway rats. European Journal of Pharmacology. 2007; 571: 215-21.
Sanderson CJ. Interleukin-5, eosinophils and disease. Blood. 1992; 79: 3101-3109.
Babre N, Debnath S, Manjunath Y, Parameshwar P, Wankhede S, Hariprasath K. Antioxidant potential of hydroalcoholic extract of Barringtonia acutangula linn roots on streptozotocin induced diabetic rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2010; 4: 201-03.
Baskar R, Lavanya R, Mayilvizhi S, Rajasekaran P. Free radical scavenging activity of antitumour polysaccharide fractions isolated from Ganoderma lucidum (Fr.) P. Karst. Natural Product Radiance. 2008; 7(4): 320-25.
Premanand R, Santhosh Kumar PH, Alladi M. Study of Thiobarbituric Reactive Substances and Total Reduced Glutathione as Indices of Oxidative Stress in Chronic Smokers with and Without Chronic Obstructive Pulmonary Disease. Indian J Chest Dis Allied Sci. 2007; 49: 9-12.
Taleb-Senouci D, Ghomari H, Krouf D, Bouderbala S, Prost J, Lacaille-Dubois MA, Bouchena M. Antioxidant effect of Ajuga iva aqueous extract in streptozotocin-induced diabetic rats. Phytomedicine. 2009; 16: 623–631.
Lowry OH, Rosenbrough NJ, Farr AC, Randell RJ. Protein measurement with folin-phenol reagent. Journal of Biological Chemistry. 1951; 193: 265–275
Qamar W, Khan R, Khan AQ, Rehman MU, Lateef A, Tahir M, Ali F, Sultana S. Alleviation of lung injury by glycyrrhizic acid in benzo(a)pyrene exposed rats:Probable role of soluble epoxide hydrolase and thioredoxin reductase. Toxicology. 2012; 291: 25-31.
Frossi B, De Carli M, Daniel KC, Rivera J, Pucillo C.Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur J Immunol. 2003; 33: 2168-77.
Barne PJ. Reactive oxygen species and airway inflammation. Free Radical Biology & Medicine. 1990; 9: 235-43.
Magi B, Bargagli E, Bini L, Rottoli P. Proteome analysis of bronchoalveolar lavage in lung diseases.Proteomics. 2006; 6: 6354–6369.
Nishida S, Teramoto K, Kimoto-Kinoshita S, Tohda Y, Nakajima S, Tomura TT, Irimajiri K. Change of Cu, Zn-superoxide dismutase activity of guinea pig lung in experimental asthma. Free Radical Research. 2002; 36: 601–606.
Ckless K, Hodgkins SR., Ather JL, Martin R, Poynter ME. Epithelial, dendritic, and CD4+ T cell regulation of and by reactive oxygen and nitrogen species in allergic sensitization. Biochim Biophys Acta. 2011; 1810: 1025–34.
Kelly HW, Sorknes CA. Asthma. In: Dipiro JT., Talbert RL., Yee GC, Matzke TR, Wells BG, Posey LM. Pharmacotherapy - A Pathophysiological Approach. Sixth ed. New York: The McGraw-Hill. 2005; 504.
Lapa JR, Bachelet CM, Pretolani M, Baker D, Scheper RJ, Vargaftig BB. Immunopathologic alterations in the bronchi of immunized guinea-pigs. Am. J. Respir Cell Mol. Biol. 1993; 9: 44-53.
Katsuyuki T, Takahiko T, Hiroki Y, Kouji F, Yukio M, Yoshinori T, Takao S. Identification and characterization of monocyte subpopulations from patients with bronchial asthma. Journal of Allergy and Clinical Immunology. 1995; 96: 230-238.
Nadeem A, Sunil K, Chhabra AM, Hanumanthrao GR. Increased oxidative stress and altered levels of antioxidants in asthma. Journal of Allergy and Clinical Immunology. 2003; 111: 72-78.
Dworski, R. Oxidant stress in asthma. Thorax. 2000; 55: 51–53.
Zhang L, Wang M, Kang X, Boontheung P, Li N, Nel AE, Loo JA. Oxidative stress and asthma: proteome analysis of chitinase-like proteins and FIZZ1 in lung tissue and bronchoalveolar lavage fluid. J Proteome Res. 2009; 8: 1631-8.
Kinnula VL, Cropo JD. Superoxide dismutase in the lung and human lung diseases. Am J Respir Crit Care Med. 2003; 167: 1600- 1619.
Jarjour NN, Calboun WJ. Enhanced production of oxygen radicals in asthma. J Lab Clin Med. 1994; 123: 131-136.
Pompella A, Visvikis A, Paolicchi A, Tata V, Casini A.The changing faces of glutathione, a cellular protagonist. Biochemical Pharmacology. 2003; 66: 1499-1503.
Kirkham P, Rahman I. Oxidative stress in asthma and COPD: Antioxidants as a therapeutic strategy . Pharmacology & Therapeutics. 2006; 111: 476-494.
Gawel S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004; 57: 453-55.
Gaetani GF, Ferraris AM, Rolfo M, Mangerini R, Arena S, Kirkman HN. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996; 87, 1595-99.
Moss DW, Henderson AR. Enzymes. In: Tietz Textbook of Clinical Chemistry, 2nd ed. Burtis CA, Ashwood ER, eds. Philadelphia: W.B. Saunders Company. 1994; 735-896.
Misaka S, Sato H, Yamauchi Y, Onoue S, Yamada S. Novel dry powder formulation of ovalbumin for development of COPD-like animal model: Physicochemical characterization and biomarker profiling in rats. European Journal of Pharmaceutical Sciences. 2009; 37: 469-476.
Moon DO, Kim MO, Lee HJ, Choi YH, Park YM, Heo MS, Kim GY. Curcumin attenuates ovalbumin-induced airway inflammation by regulating nitric oxide. Biochemical and Biophysical Research Communications. 2008; 375: 275-79.
Diaz P, Gonzalez MC, Gallenguillos F. Leukocytes and mediators in bronchoalveolar lavage during allergen induced late-phase asthmatic reactions. Am. Rev. Respir Dis. 1989; 139: 1383-88.
Henderson RF, Muggenburg BA. Use of Bronchoalveolar Lavage to Detect Lung Injury. Current Protocols in Toxicology. 2004; 21: 265–287.
Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem. Pharmacol. 1999; 58: 759-765.
Brightling CE, Bradding P, Symon, FA, Holgate ST, Wardlaw AJ, Pavord I, Chang L, Crapoa JD. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radical Biology and Medicine. 2002; 33: 379–86.
Lee Ju-Young Jung, Kyoung-youl, Mee-Young Lee, Dayoung Jung, Eun-Sang Cho, Hwa-Young Son. Antioxidant and antiasthmatic effects of saucerneol D in a mouse model of airway inflammation. International Immunopharmacology. 2011; 11: 698-705.
Jung WS, Chung IM, Kim SH, Kim MY, Ahmad A, Praveen N. In vitro antioxidant activity, total phenolics and flavonoids from celery (Apium graveolens) leaves. Journal of Medicinal Plants Research. 2011; Vol. 5(32): pp. 7022–7030, 30.
Kawabata Y, Aoki Y, Matsui T, Yamamoto K, Sato H, Onoue S, Yamada S . Stable dry powder inhaler formulation of tranilast attenuated antigen-evoked airway inflammation in rats. European Journal of Pharmaceutics and Biopharmaceutics. 2011; 77: 178-181.
Carlo GD., Mascolo N, Lzzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sciences. 1999; 65: 337-53.
Geetha VS, Visthwanathan S, Kameshwaren L. Comparison of total alkaloids of Tylophora indica and disodium cromoglycate on mast cell stabilization. Indian J Pharmacol. 1981; 13: 199-201.
Vijayalakshmi CR, Velraj MH & Jayakumari. Antianaphylactic and Anti-Inflammatory Activities of a Bioactive Alkaloid from the Root Bark of Plumeria acutifolia Poir. Iranian J Pharm Res 2011; 10: 525-533.
Zhang L, Wang M, Kang X, Boontheung P, Oxidative Stress and Asthma: Proteome Analysis of Chitinase-like Proteins and FIZZ1 in Lung Tissue and Bronchoalveolar Lavage Fluid. J Proteome Res. 2010; April 1: 1-20.
Chang L, Crapoa JD. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radical Biology & Medicine. 2002; 33: 379–86.
Ni Y, Jian Wang, Xiao-Long Yan, Feng Tian, Jin-Bo Zhao, Yun-Jie Wang, Tao Jiang. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respiratory Research 2010, 11:33, 1-8.