CYCLOPHOSPHAMIDE ALTERED THE MYOCARDIAL MARKER ENZYMES: PROTECTION PROVOKED BY HESPERIDIN IN RATS
Keywords:
Antioxidants, Cardiotoxicity, Cyclophosphamide, Hesperidin, Oxidative stressAbstract
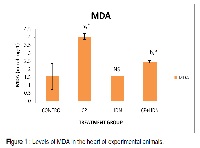
Our present experimentation was carried out to evaluate the efficacy of hesperidin (HDN) (100mg/kg body weight), administered orally for 7 days on cyclophosphamide (CP) elicited oxidative damage on rat heart. Cardiotoxicity inflicted by single intraperitoneal injection of CP (200mg/kg body weight) was manifested by exalted levels of CPK (creatinine phosphokinase),ALT (alanine transaminase), AST (aspartate transaminase) and LDH (lactate dehydrogenase).CP induced group depicted significant amelioration in level of MDA (malondialdehyde) , HDN treated group demonstrated inflated levels of above enzymes and decreased level of MDA. These levels were reversed to normal levels in HDN treated group. Thus result of our study is in concordance with the notion that HDN is adept in combating myocardial free radical damage provoked by CP thus proving its protective potential.
References
Abraham P, Sugumar E. Increased glutathione levels and activity of PON1 (phenyl acetate esterase) in the liver of rats after a single dose of cyclophosphamide a defense mechanism. Exp Toxicol Pathol. 2008, 59(5): 301-6.
Ahmadi A, Hosseinimehr S.J, Naghshvar F, Hajir E, Ghahremani M. Chemoprotective effects of hesperidin against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Arch Pharm Res. 2008, 31(6): 794-7.
Amy T, Jennifer Wo, Caron J, Aaron Weitzman, Tamara Horwich, Charles Hesdorffer, David Savage, Andrea Troxel. Cardiac toxicity observed in association with high dose Cyclophosphamide – based chemotherapy for metastatic breast cancer. The Breast. 2004 , 13: 341-346.
Annida Balakrishnan, Venugopal P, Menon. Effect of hesperidin on matrix metalloproteinases and antioxidant status nicotine – induced toxicity. Toxicology. 2007, 238: 90-98.
Broad J, Sirota, J H. Renal clearance of endogenous creatinine in man. J. Clin Invest. 1948, 27: 646.
Lushnikova E L, Tolstikova T G, Nepomnyashchikh L M, Klinnikova M G, Mol odykh O P, Sviridov E A, Sorokina I V, and Zhukova N A. Cardiomyocyte count in rat myocardium under the effect of antitumor agents cyclophosphamide and triterpenoids. Bulletin of Experimental Biology and Medicine. 2007, 144: 3.
Willis E D. Effects of lipid peroxidation on membrane – bound enzymes of the Endoplasmic reticulum. Biochem J. 1971, 123: 983- 991.
Elangovan Selvakumar, Chidambaram Prahalathan, Periyasamy Thandavan Sudharshan, Palaninathan Varalakshmi. Protective effect of lipoic acid on Cyclophosphamide induced testicular toxicity. Clinica Chimica Acta. 2006, 367: 114-119.
Gaganjit Kaur, Naveen Tirkey, Kanwaljit Chopra. Beneficial effect of hesperidin on lipopolysaccharide-induced hepatotoxicity. Toxicology. 2006, 226: 152-160.
Grafstrom R C, Dypbukt J M, Willey J C, Sundqvist K, Edman C, Atzori L, and Harris C C. Pathobiological effects of acrolein in cultured human bronchial epithelial cells. Cancer Res. 1988, 48: 1717–1721.
Hirano A, Shimizu T, Watanabe O, Kinoshita J, Kimura K, Kamimura M, Domoto K, Aiba M, Ogawa K. Epirubicin and cyclophosphamide followed by docetaxel as primary systemic chemotherapy in locally advanced breast cancer. Anticancer Res. 2008, 28(6B): 4137-42.
Van Vleet, J F, and Ferrans, V J. Myocardial diseases of animals. American Journal of Pathology. 1986, 124: 98-178.
John M P, Frederick R A, Janet F E, Joseph R, Darrell R F, Ted G, Katherine R, Eneida N, Eileen S, Larry D, Jeanette C, Mary M H, Oliver W P, Ajay K G, Paul J M, Irwin D B and Dana C Matthews. 131I–anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2005, 107(5): 2184–2191.
Kannampalli Pradeep, Sang Hyun Park, Kyong Cheol Ko. Hesperidin a flavanoglycone protects against gamma-irradiation induced hepatocellular damage and oxidative stress in Sprague-Dawley rats. European journal of pharmacology. 2008, 587: 273-280.
Kern Julie C, Kehrer James P. Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chemico-biological interactions. 2002, 139: 79-95.
King J. The dehydrogenase or oxidoreductase-lactate dehydrogenase. In: Practical clinical enzymology Van D (ED). Nostrand Company Limited, London, 1965a, 83-93.
Kirsten J M, Schimmel Dick J, Richel, Renee B A, Van den Brink, Henk-Jan Guchelaar. Cardiotoxicity of cytotoxic drugs. Cancer treatment reviews. 2004, 30: 181-191.
Lindley C M G, Hamilton J S, McCune S, Faucette S S, Shord R L, Hawke H, Wang Gilbert D S, Jolley B Yan and Lecluyse E L. The effect of Cyclophosphamide with and without dexamethasone on cytochrome p450 3A4 and 2B6 in human hepatocytes. Drug Metabolism and Disposition. 2002, 30: 814-822.
Gharib M I, Burnett A K. Chemotherapy-induced cardiotoxicity: current practice and prospects of prophylaxis. European Journal of Heart Failure. 2002, 4: 235-242.
Murgo A J, Weinberger B B. Pharmacological bone marrow purging in autologous transplantation: focus on the cyclophosphamide derivatives. Crit Rev Oncol Hematol. 1993, 14(1): 41-60.
Mythili, Y., Sudharsan, P.T., Selvakumar, E., Varalakshmi, P., Protective effect of DL- alpha-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem Biol Interact. 2004, 151(1): 13-9.
Naveen Tirkey, Sangeeta Pilkhwal, Anurag Kuhad and Kanwaljit Chopra. Hesperidin, a citrus bioflavinoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and klidney. BMC Pharmacology. 2005, 5: 2.
Ohkawa H, Ohishi N,Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979, 95: 351-358.
Patel J M, Block E R. Cyclophosphamide-induced depression of the antioxidant defense mechanisms of the lung. Exp Lung Res.1985, 8(2-3): 153-65.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957, 28: 56-63.
Serra H, Mendes T, Bronze M R, Simplício A L. Prediction of intestinal absorption and metabolism of pharmacologically active flavones and flavanones. Bioorg Med Chem. 2008, 16(7): 4009-18.
Subramanian Senthlikumar, Thiruvengadam Devaki, Bhakthavatchalam Murali Manohar, Munismay Suresh Babu. Effect of squalene on Cyclophosphamide induced toxicity. Clinica Chimica Acta.. 2005, 364: 335-342.
Sudharsan, Periyasamy Thandavan, Mythili, Yenjerla, Selvakumar, Elangovan,Varalakshmi, Palaninathan. Lupeol and Its Ester Exhibit Protective Role against Cyclophosphamide-Induced Cardiac Mitochondrial Toxicity. Journal of Cardiovascular Pharmacology. 2006, 47(2): 205-210.
Un Ju Jung, Mi-Kyung Lee, Kyu-Shik Jeong and Myung-Sook Choi. The Hypoglycemic Effects of Hesperidin and Naringin Are Partly Mediated by Hepatic Glucose-Regulating Enzymes in C57BL/KsJ-db/db Mice. The American Society for Nutritional Sciences J. Nutr. 2004, 134: 2499-2503.
Wilmsen P K, Spada D S, Salvador M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J Agric Food Chem. 2005, 53(12): 4757-61.
Yagi K. A simple fluorometric assay for lipid peroxide in blood plasma. Biochem Med. 1976, 15: 212-216.
Zhang Jing, Tian Quan, Zhou Shu-Feng. Clinical Pharmacology of Cyclophosphamide and Ifosfamide. Current Drug Therapy. 2006, 1: 55- 84(30).