Protective effects of Origanum majorana L. against Neurodegeneration: Fingerprinting, Isolation and In vivo Glycine Receptors Behavioral Model
Keywords:
Phytotherapy, Glycine receptors, Origanum majorana L., HPLC fingerprinting, Synergism, Neurodegenerative disordersAbstract
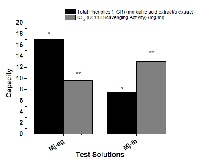
Extracts from Origanum majorana L. are used in Asia and Africa as sedatives and neurotonics. Few studies done to explore the active chemical constituents responsible for this apparent contrast. The inhibitory glycine receptors (GlyRs) are key mediators of synaptic signaling in spinal cord, brain stem, and higher central nervous system regions. Neurodegeneration may cause alteration of the GlyRs causing strychnine-like convulsions and stiffness. Here, modulation of GlyRs in-vivo was studied in a mouse model of strychnine toxicity. Total phenolics in Egyptian marjoram (Mj-eg) and Lebanese marjoram(Mj-lb) were calculated and fingerprinted. The Mj-eg and Mj-lb ethanolic extracts revealed to be potent modulators to GlyR; with potential anticonvulsant properties in low doses. Chlorogenic acid (CGA), p-coumaric acid (pCA) and p-hydroxy benzoic acid (pHBA) were detected in the active fractions via column chromatography and RP-HPLC fractionation. The active fraction phenolics at harmless low doses, showed anticonvulsant activity by reversing strychnine toxicity in mice. By applying Berenbaum isoblographic method, we confirmed that at low concentrations the protective effects of CGA and pCA on strychnine toxicity are synergistic. It could be concluded that both marjoram and active phenolics could be used as sedatives in low doses and as neurotonics in high doses. In order to fight against neurodegenerative diseases is to improve body antioxidant, marjoram provided to be good sources for antioxidant potential. In brief, the marjoram phenolics, especially CGA, pCA and pHBA, suggested to be novel GlyR modulators, good phytotherapy, pharmacological tool and a dose sensitive drug to treat convulsions, stiffness and neurodegenerative disorders.
References
Khan, IA, Abourashed, EA, Leung's Encyclopedia of Common Natural Ingredients: Used in Food, Drugs and Cosmetics, 3rd ed., Wiley, United States; 2009. p. 810.
. Hossain, B, Rai, K, Brunton, P, Martin-Diana, B, Barry-Ryan, C: Characterization of phenolic compounds in marjoram (Origanum majorana L.) by LC-ESI-MS/MS.: In Proceedings of the International conference of food innovation (foodinnova) 25-29 October 2010; Spain. Edited by Fito, P: Toldra, F; 2010: 408.
. Stevenson E, Hurst D, Polyphenolic phytochemicals-just antioxidants or much more?, Cell Mol Life Sci. 2007; 64(22): 2900-2916.
. Kandaswami C, Middleton E, Free radical scavenging and antioxidant activity of plant flavonoids, J Adv. Exp. Med. Biol. 1994; 366(1): 351-376.
. Kandaswami C, Perkins E, Drzewiecki G, Soloniuk S, Middleton E, Differential inhibition of proliferation of human squamous cell carcinoma, gliosarcoma and embryonic fibroblast-like lung cells in culture by plant flavonoids, Anticancer Drugs. 1992; 3(5): 525-530.
. Middleton E, Kandaswami C, Theoharides C, The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer, Pharmacol Rev. 2000; 52(4): 673-751.
. Harborne, B, Williams A, Advances in flavonoid research since 1992, Phytochemistry. 2000; 55(6): 481-504.
. Griebel G, Perrault G, Tan S, Schoemaker H, Sanger J, Pharmacological studies on synthetic flavonoids: comparison with diazepam, J Neuropharmacology. 1999; 38(7): 965-977.
. Singleton L, Orthofer R, Lamuela-Raventós, M, Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent, J. Methods in Enzymology. 1999; 299(1): 152–178.
. Singleton L, Rossi A, Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents, Am. J. Enol. Vitic.1965; 16(3): 144-158.
. Clifford N, Chlorogenic acids and other cinnamates – nature, occurrence and dietary burden, Journal of the Science of Food and Agriculture. 1999; 79(3): 362–372.
. Kozłowska M, Laudy E, Starościak J, Napiórkowski A, Chomicz L, Kazimierczuk Z, Antimicrobial and Antiprotozoal Effect of Sweet Marjoram (Origanum marjorana L.), Acta Sci. Pol. 2010; 9(4): 133-141.
. Stacewicz-Sapuntzakis M, Bowen, E, Hussain A, Damayanti-Wood I, Farnsworth R, Chemical composition and potential health effects of prunes: a functional food?, Crit Rev Food Sci Nutr. 2001;41(4): 251-286.
. Hossain B, Catherinea, B-R, Martin-Diana B, Brunton P, Optimisation of Accelerated Solvent Extraction of Antioxidant Compounds from Rosemary (Rosmarinus officinalis L.), Marjoram (Origanum majorana L.) and Oregano (Origanum vulgare L.) Using Response Surface Methodology, Food Chemistry. 2011; 126(1): 339–346.
. Gálvez C, Barroso G, Pérez-Bustamante A, Analysis of polyphenolic compounds of different vinegar samples, European Food Research and Technology. 1994; 199(1): 29-31.
. Lee J, Talcott S, Fruit maturity and juice extraction influences ellagic acid derivatives and other antioxidant polyphenolics in muscadine grapes, Journal of Agricultural and Food Chemistry. 2004; 52(1): 361-6.
. Andarwulan N, Kurniasih D, Apriady A, Rahmat H, Rotoc V, Bolling W, Polyphenols, carotenoids, and ascorbic acid in underutilized medicinal vegetables. , Journal of Functional. 2012; 4(1): 339-347.
. Liu Z, Ou S, Huang X, Luo Y, Xie J, Xu H, Extraction of ferulic acid from enzymatic hydrolysate by ion-exchange adsorption, Zhong Yao Cai. 2004; 27(12): 910-913.
. Labaune P, Moreau P, Byrne R, Comparative physiological disposition of two nitrofuran anti-microbial agents, Biopharm Drug Dispos. 1986; 7(1) 431-441.
. Vanhoof R, Hubrechts M, Roebben E, Nyssen J, Nulens E, Leger J, The comparative activity of pefloxacin, enoxacin, ciprofloxacin and 13 other antimicrobial agents against enteropathogenic microorganisms, Infection. 1986; 14(6): 294-298.
. Peungvicha P, Temsiririrkkul R, Prasain K, Tezuka Y, Kadota S, Thirawarapan S, 4-Hydroxybenzoic acid: a hypoglycemic constituent of aqueous extract of Pandanus odorus root, J Ethnopharmacol. 1998; 62(1): 79-84.
. Di Matteo V, Pierucci M, Di Giovanni G, Di Santo A, Poggi A, Benigno A, Aspirin protects striatal dopaminergic neurons from neurotoxin-induced degeneration: an in vivo microdialysis study, Brain Res. 2006; 1095(1): 167-177.
. Raafat K, Breitinger U, Mahran L, Ayoub N, Breitinger H-G, Synergistic Inhibition of Glycinergic Transmission In Vitro and In Vivo by Flavonoids and Strychnine, Toxicological Sciences. 2010; 118(1) 171–182.
. Lynch W, Native glycine receptor subtypes and their physiological roles Neuropharmacology, J Neuropharmacology. 2009; 56(1): 303-09.
. Becker L, von WegererJ, Schenkel, J., Zeilhofer, H.U., Swandulla, D., Weiher, H., Disease-specific human glycine receptor alpha1 subunit causes hyperekplexia phenotype and impaired glycine- and GABA(A)-receptor transmission in transgenic mice, J Neurosci. 2002; 22(7) 2505-2512.
. Chindo A, Anuka A, McNeil L, Yarob H, Adamu S, Amos S, Anticonvulsant properties of saponins from Ficus platyphylla stem bark, Brain Research Bulletin. 2009; 78 (1): 276-282.
. Berenbaum C, Synergy, additivism and antagonism in immunosuppression. A critical review, Clin Exp Immunol. 1977; 28(1): 1-18.
. Wagner H, Ulrich-Merzenich G, Synergy research: approaching a new generation of phytopharmaceuticals, Phytomedicine. 2009; 16(2-3) 97-110.
. Kogure K, Yamauchi I, Tokumura A, Kondou K, Tanaka N, Takaishi Y, Novel antioxidants isolated from plants of the genera Ferula, Inula, Prangos and Rheum collected in Uzbekistan, Phytomedicine. 2004; 11(7-8) 645-651.
. Mavi A, Terzi Z, Ozgen U, Yildirim A, Coskun M, Antioxidant properties of some medicinal plants: Prangos ferulacea (Apiaceae), Sedum sempervivoides (Crassulaceae), Malva neglecta (Malvaceae), Cruciata taurica (Rubiaceae), Rosa pimpinellifolia (Rosaceae), Galium verum subsp. verum (Rubiaceae), Urtica dioica (Urticaceae), Biol Pharm Bull. 2004; 27(5): 702-705.
. Ahmed J, Guvenc A, Kucukboyaci N, Balbemir A, Coskun M, Total phenolic contents and antioxidant activities of Prangos Lindl. (Umbelliferae) species growing in Konya province (Turkey), Turk J Biol. 2011; 35(1): 353-360.
. Baravalia Y, Kaneria M, Vaghasiya Y, Antioxidant and antimicrobial activity of Diospyros ebenum Roxb. Leaf extracts, Turk J Biol. 2009; 33(1): 159-164.
. Slinkard K, Singleton L, Total phenols analysis: automation and comparison with manual methods, Am J Enology Viticulture. 1977; 28(1): 49-55.
. Moral R, On the variability of chlorogenic acid concentration, Oecologia. 1972; 9(3): 289-300.
. Devi S, Kumar S, Shankar S, Effect of purification (Suthi) on the acute toxicity of seeds of Nux-vomica, J Pharmacology and Toxicology. 2011; 1(1): 38-42.
. Setnikar I, Murmann W, Magistretti J, Da Re P, Amino-methylchromones, brain stem stimulants and pentobarbital antagonists, J Pharmacol Exp Ther. 1960; 128(1): 176-181.
. Basha, AIU. 11 : siyar, al-Tabah 1. ed., Dar al-Farabi, Bayrut, 2006. p. 547.
. Global Book Publishing (Firm), Beitzel, B.J., Whiddon, L., Biblica : the Bible atlas : a social and historical journey through the lands of the Bible, Barron's Educational Series,, Hauppage, NY, 2007. p. 354.
. Kwon H, Lee K, Kim A, Hong I, Kim C, Jo H, Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice, Eur J Pharmacol. 2010: 649(1-3): 210-217.
. Céspedes L, Valdez-Morales M, Avila G, El-Hafidi M, Alarcón J, Paredes-López O, Phytochemical profile and the antioxidant activity of Chilean wild black-berry fruits, Aristotelia chilensis (Mol) Stuntz (Elaeocarpaceae), Food Chemistry. 2010; 119(1): 886-895.
. Ding Y, Extracts and Constituents of Rubus chingii with 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Free Radical Scavenging Activity, Int J Mol Sci. 2011; 12(6): 3941-3949