Preliminary phytochemical screening and Bioactivity of selected Indian Medicinal plants
Keywords:
Medicinal plants, methanol extracts, skin diseases, antibacterial agents, antioxidantsAbstract
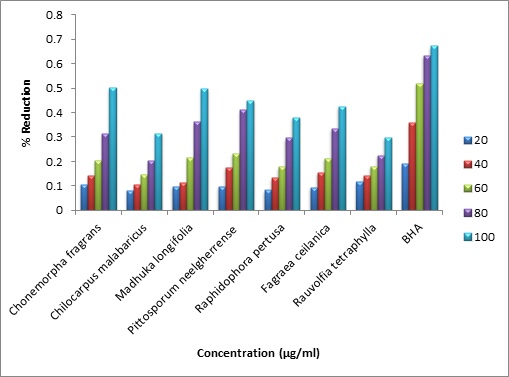
The methanolic crude extracts of Chonemorpha fragrans (Moon), Chilocarpus malabaricus Bedd Madhuka longifolia (Koenig) J.F.Macbr, Pittosporum neelgherrense Wightt, Raphidophora pertusa (Roxb.) Schott, Fagraea ceilanica Thunb and Rauvolfia tetraphylla L. were screened for the presence of phytoconstituents and their ability to possess antimicrobial and free radical scavenging ability using Chloramphenicol, The methanolic crude extracts of Chonemorpha fragrans (Moon) Alston, Chilocarpus malabaricus Bedd., Madhuka longifolia (Koenig) J.F.Macbr., Pittosporum neelgherrense Wightt., Raphidophora pertusa (Roxb.) Schott., Fagraea ceilanica Thunb., and Rauvolfia tetraphylla L., were screened for the presence of phyto-constituents and their ability to possess antimicrobial and free radical scavenging ability using chloramphenicol, cephoperazone and ascorbic acid as respective standards. Antimicrobial activity was evaluated by Kirby-Bauer disc diffusion method and free radical scavenging activity was evaluated using 1,1-diphenyl-2-picryl hydrazyl (DPPH) free radical and reducing power assay. Pittosporum neelgherrense showed better overall antimicrobial activity and Madhuka longifolia proved better antioxidant ability possessing low IC50 valueof30 µg/ml compared to the other selected medicinal plants. The highest total phenol content was found to be in Chonemorpha fragrans with the value 88±0.121mg/g. The present study reveals that the selected plants serve as a source of antimicrobial and antioxidant drugs in future and thus, can find applications in food and pharmaceutical industries.
References
. Sumitra Chanda, Yogesh Baravalia. Screening of some plant extracts against some skin diseases caused by oxidative stress and Microorganisms. African Journal of Biotechnology 2010; 9(21): 3210-3217.
. Cerutti P, Shah G, Peskin A, Amstad P. Oxidant carcinogenesis and antioxidant defense. Ann NY AcadSci 1992; 663: 158–66.
. Motamed SM, Naghibi F. Antioxidant activity of some edible plants of the Turkmen Sahra region in northern Iran. Food Chem 2010; 119: 1637-1642.
. Myachi Y. Skin diseases associated with oxidative injury, In: Fuchs J, Parcker L (ed). Oxidative Stress in Dermatology. Marcel Dekker, New York, 1993, pp. 323-331.
. David R, Bickers, Mohammad Athar. Oxidative Stress in the Pathogenesis of Skin Disease, Journal of Investigative Dermatology 2006; 126: 2565-2575.
. Krishnaraju AV, Rao TVN, Sundararaju D. Assessment of bioactivity of Indian Medicinal Plants using Brine shrimp (Artemiasalina) lethality assay. Int J ApplSciEng 2005; 2: 125-134.
. O guyemi AO. In: SofawaraA.ed Proceedings of a conference of African medicinal plants. Ife-Ife: Univ life, 1979, pp. 20-22.
. Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disc method. American Journal of Clinical Pathology 1966; 45: 493-496.
. Singleton VL, Rossi JA. Colorimetry of total phenolic substances. US: American Chemical Society Symposium series. 1965; 26: 47-70
. Sunil K, Dinesh K,Manjusha, Kamal S, Nidhan S,Bhoodev V. Antioxidant and free radical scavenging activity of Citrulluscolocynthis(L.) Schrad. Methanolic fruit extract. Acta pharm 2008; 58 :215-220.
. Lu Y, Foo Y. Antioxidant activities of polyphenols from sage (Salvia officinalis.) Food Chem2000; 75: 197-202.
. Adedapo AA, Mogbojuri OM, Emikpe BO. Safety evaluations of the aqueous extract of the leaves of Moringaoleifera.J Med Plants Res 2000;3(8):586–591.
. Callow RK. Steroids. Proc. Royal, Soc London Series A, 1936, pp.157-194.
. Scalbert A. Antimicrobial properties of tannins. Phytochemistry, 1991, 30, pp. 3875-3883.
. Field JA, Lettinga G. Toxicity of tannic compounds to microorganisms. Plants Polyphenols. Synthesis, Properties, Significance. Basic Life Science 1992; 59: 673-692.
. Hakkim FL, Shankar CG, Girija S. Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems and inflorescence and their in vitro calluscultures. J Agri and Food Chem 2007;55: 9109–9117.
. Chen CW, Ho CT. Antioxidant properties of polyphenols extracted from green tea and black tea. Journal of Food Lipids1995; 2: 35-46.