Potential of Cassia auriculata and Saraca asoca standardized extracts and their principal components for alleviating diabetic complications
Keywords:
aldose reductase, advanced glycation endproducts, galactitol accumulation, Cassia auriculata, Saraca asoca, proanthocyanidin B1Abstract
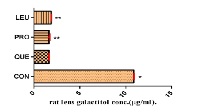
Aldose reductase (AR) enzyme and advanced glycation endproducts (AGEs) play an important role in diabetic complications such as cataracts. The purpose of this study was to look into two standardized plant extracts used in Ayurvedic medicine for the treatment of diabetes, and their principal components for AR and AGEs inhibitory activities, and to evaluate their potential in combating the various pathological consequences of diabetes. Cassia auriculata Linn and Saraca asoca (Roxb.) De Wild and their respective major constituents, proanthocyanidin B1, and leucocyanidin were studied for their inhibitory activity against rat lens AR (RLAR), rat kidney AR (RKAR), human recombinant AR (HRAR), and generation of AGEs. In addition, in vivo inhibition of lens galactitol accumulation by the major constituents of the plants in galactose-fed rat model has been studied. The results show that both plant extracts and their principal components possess AR inhibitory actions in both in vitro and in vivo assays, and also inhibited AGEs formation significantly. In all assays carried out, proanthocyanidin B1 was found to be the most potent showing comparable or better effect than the reference compounds used. In both RP-HPLC and GLC analyses, rat lens galactitol concenration of procyanidin B1 ( 1.5, 1.6 mg/ml, respectively) displayed a slightly better activity than the reference compound quercetin (1.65, 1.63 mg/ml, respectively). The results obtained in this study give a new dimension to the hitherto unknown activity of the plants as possible protective agents against long-term diabetic complications.
References
Hung HY, Qian K, Morris-Natschke SL, Hsu CS, Lee KH. Recent discovery of plant-derived antidiabetic natural products. Nat Prod Rep 2012;29:580–606.
Rao AR, Veeresham C, Asres K. In Vitro and In Vivo Inhibitory Activities of Four Indian Medicinal Plant Extracts and their Major Components on Rat Aldose Reductase and Generation of Advanced Glycation Endproducts. Phytother Res, in press.
Ramirez MA, Borja NL. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy 2008;28:646-55.
Jung HA, Jung YJ, Yoon NY, Jeongda M, Bae HJ, Kim DW, Na DH, Choi JS. Inhibitory effects of Nelumbo nucifera leaves on rat lens aldose reductase, advanced glycation endproducts formation, and oxidative stress. Food chem toxicol 2008;46:3818–3826.
Joshi SG, Cesalpinaceae - Cassia auriculata. Text Book of Medicinal Plants. pp 119, 2000.
Pari L, Latha, M. Effect of Cassia Auriculata flowers on blood sugar levels, serum and tissue lipids in streptozotocin diabetic rats. Singapore Medical Journal 2002;43:617–621.
Sunil K, Smita N, Dinesh K, Gurvirender S, Sumit N, Renu A. Evaluation of antihyperglycemic and antioxidant activities of Saraca asoca (Roxb.) De Wild leaves in streptozotocin induced diabetic mice. Asian Pacific Journal of Tropical Disease 2012;2:170–176.
Hayman S, Kinoshita JH. Isolation and properties of lens aldose reductase. J Biol Chem 1965;240:877–882.
Chethan S, Dharmesh SM, Malleshi NG. Inhibition of aldose reductase from cataracted eye lenses by finger millet (Eleusine coracana) polyphenols. Bioorg Med Chem 2008;16:10085–10090.
Lowry OH, Rosebrogh NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265–275.
Cerelli MJ, Curtis DL, Dunn JP, Nelson PH, Peak TM, Waterbury LD. Antiinflammatory and aldose reductase inhibitory activity of some tricyclic arylacetic acids. J Med Chem 1986; 29:2347–2351.
Nishimura C, Yamaoka T, Mizutani M, Yamashita K, Akera T, Tanimoto T. Purification and characterization of the recombinant human aldose reductase expressed in baculovirus system. Biochim Biophys Acta 1991;1078:171–178.
Vinson JA, Howard TB. Inhibition of protein glycation and advanced glycation end product by ascorbic acid and other vitamins and nutrients. J Nutr Biochem 1996;7:659–663.
Kato A, Higuchi Y, Goto H, Kizu H, Okamoto T, Asano N, Hollinshead J, Nash RJ, Adachi I. Inhibitory effects of Zingiber officinale Roscoe derived components on aldose reductase activity in vitro and in vivo. J Agric Food Chem 2006;54:6640–6644.
. Dethy JM, Callaert-Deveen B, Janssens M, Lenaers A. Determination of sorbitol and galactitol at the nanogram level in biological samples by high-performance liquid chromatography. Anal Biochem 1984;143:119–124.
Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820.
Kato A, Yasuko H, Goto H, Hollinshead J, Nash RJ, Adachi I. Inhibitory effect of rhetsinine isolated from Evodia rutaecarpa on aldose reductase activity. Phytomedicine 2009;16:258–261.
Peyroux, J., and Sternberg, M. Advanced glycation end products (AGEs): pharmacological inhibition in diabetes. Pathologie Biologie 2006;54:405–419.
Collier M, Small M. The role of the polyol pathway in diabetes mellitus. British Journal of Hospital Medicine 1991;45:38–40.
Nicolucci A, Carinci F, Cavaliere D, Scorpiglione N, Belfiglio M, Labbrozzi D, Mari E, Benedetti MM, Tognoni G, Liberati AA. A meta-analysis of trials on aldose reductase inhibitors in diabetic peripheral neuropathy. The Italian Study Group. The St. Vincent Declaration. Diabet Med 1996;13:1017–1026.
Jung HA, Yoon NY, Kang SS, Kim YS, Choi JS. Inhibitory activities of prenylated flavonoids from Sophora flavescens against aldose reductase and generation of advanced glycation endproducts. J Pharm Pharmacol 2008;60:1227–1236.
Wirasathien L, Pengsuparp T, Suttisri R, Ueda H, Moriyasu M, Kawanishi K. Inhibitors of aldose reductase and advanced glycation end- products formation from the leaves of Stelechocarpus cauliflorus R.E.Fr. Phytomedicine 2007;14:546–550.
Eun HL, Dae-Geun S, Joo YL, Cheol-Ho P, Byung HU, Sang HJ. Inhibitory effect of the compounds isolated from Rhus verniciflua on aldose reductase and advanced glycation endproducts. Biol Pharma Bull 2008;31:1626–1630.
Heynengen RV. Formation of polyols by the lens of the rat with sugar cataract. Nature 1959;184:194.