28-Day Repeated Dose Oral Toxicity of a Herbal Mixture Dia-2, Containing Standardized Extracts of Allium Sativum and Lagerstroemia Speciosa In Sprague Dawley Rats
Keywords:
Allium sativum, Lagerstroemia speciosa, diabetes mellitus, repeated oral toxicity, herbal formulationAbstract
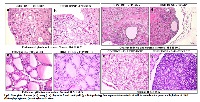
Allium sativum [ASE] and Lagerstroemia speciosa [LSE] are widely used in folk medicine as a medication for diabetes. DIA-2 is a polyherbal antidiabetic formulation containing fixed combination [1:1 w/w] of standardized aqueous extracts of Allium sativum bulbs containing 1.1 % alliin w/w and 40 % hydroalcholic extract of Lagerstroemia speciosa leaves containing 1.28% w/w corosolic acid. Earlier studies in our laboratories have demonstrated the oral safety of DIA-2 on acute oral exposure to female Sprague Dawley [SD] rats and the antidiabetic activity of DIA-2 in high-fat diet fed/streptozotocin-induced diabetic rats. The ingredients of DIA-2 have long history safety but however, there is little toxicological information regarding the oral safety on repeated exposure of ASE and LSE when given as a combined mixture. The present study evaluated the repeated oral toxicity of DIA-2 in both the sexes of SD rats. Rats were treated orally once with 62.5, 125, 250 mg/kg body weight, and animals were observed till the 28 days of study. On repeated oral administration, DIA-2 showed did not exhibit any clinical signs of toxicity, mortality, significant change in food, water consumption, body weight, mortality, clinical chemistry, hematology, organ weight, gross pathology and histopathology when varying doses of the DIA-2 were administered orally once daily for a period of 28 days. The NOAEL [No Observed Adverse Effect Level] of DIA-2 in this study was identified to be greater than 250 mg/kg/day. The results from the study suggest that there are no toxicologically significant effects on 28 day repeated oral administration of DIA-2 and the data also provide satisfactory preclinical evidence on its oral safety to support its use as a therapeutic agent in the treatment of diabetes mellitus.
References
. Zimmet P, Boyko EJ, Collier GR, de
Courten M. Etiology of the metabolic
syndrome: potential role of insulin
resistance, leptin resistance, and
other players. Ann N Y Acad Sci.
; 892:25-44.
. Amin KA, Nagy MA. Effect of
Carnitine and herbal mixture extract
on obesity induced by high fat diet in
rats. Diabetol Metab Syndr. 2009;
:17.
. Kelly GS. Insulin resistance: lifestyle
and nutritional interventions. Altern
Med Rev. 2000; 5:109-132.
. Medina-Gomez G, Gray S, VidalPuig A. Adipogenesis and
lipotoxicity: role of peroxisome
proliferator-activated receptor
gamma [PPARgamma] and
PPARgammacoactivator-1 [PGC1].
Public Health Nutr. 2007;10:1132-
. Klein G, Kim J, Himmeldirk K, Cao Y,
Chen X. Antidiabetes and Antiobesity Activity of Lagerstroemia
speciosa. Evid Based Complement
Alternat Med. 2007; 4:401-407.
. Das SK, Chakrabarti R. Non-insulin
dependent diabetes mellitus: present
therapies and new drug targets. Mini
Rev Med Chem. 2005; 5:1019-1034.
. Gupta R, Bajpai KG, Johri S, Saxena
AM. An overview of Indian novel
traditional medicinal plants with antidiabetic potentials. Afr J Tradit
Complement Altern Med. 2007; 5:1-
. Liu, C.T., Sheen, L.Y., Lii, C.K.,
Does garlic have a role as an
antidiabetic agent? Mol Nutr Food
Res. 2007; 51: 1353-1364.
. Ambati S, Yang JY, Rayalam S, Park
HJ, Della-Fera MA, Baile CA. Ajoene
exerts potent effects in 3T3-L1
adipocytes by inhibiting
adipogenesis and inducing
apoptosis. Phytother Res. 2009;
:513-518.
. Bai, N., He, K., Roller, M., Zheng, B.,
Chen, X., Shao, Z., Peng, T., Zheng,
Q. Active compounds from
Lagerstroemia speciosa, insulin-like
glucose uptake-stimulatory/inhibitory
and adipocyte differentiationinhibitory activities in 3T3-L1 cells. J
Agric Food Chem. 2008; 56: 11668-
. Tiwari AK and Rao JM: Diabetes
mellitus and multiple therapeutic
approaches of phytochemicals:
Present status and future prospects.
Current Sci; 2002; 83:30-37.
. Alnaqeeb MA, Thomson M, Bordia T,
Ali M. Histopathological effects of
garlic on liver and lung of rats.
Toxicol Lett. 1996; 85:157-164.
. Morcos NC, Camilo K. Acute and
chronic toxicity study of fish oil and
garlic combination. Int J Vitam Nutr
Res. 2001; 71:306-312.
. Banerjee SK, Maulik M, Manchanda
SC, Dinda AK, Das TK, Maulik SK.
Garlic-induced alteration in rat liver
and kidney morphology and
associated changes in endogenous
antioxidant status. Food Chem
Toxicol. 2001; 39:793-797.
. Mikail HG. Phytochemical screening,
elemental analysis and acute toxicity
of aqueous extract of Allium sativum
L. bulbs in experimental rabbits.
Journal of Medicinal Plants
Research. 2010; 4: 322-326.
. Stohs SJ, Miller H, Kaats GR. A
Review of the Efficacy and Safety of
Banaba [Lagerstroemia speciosa L.]
and Corosolic Acid. Phytother Res.
;26:317-324.
. Kesavanarayanan KS, Sathiya S,
Kalaivani P, Ranju V, Sunil AG,
Saravana Babu C, Kavimani S,
Prathiba D. DIA -2, a polyherbal
formulation ameliorates
hyperglycemia and protein-oxidation
without increasing the body weight in
Type II diabetic rats. Eur Rev Med
Pharmacol Sci. 2012 [Article in
press].
. Perera PK, Li Y. Functional herbal
food ingredients used in type 2
diabetes mellitus. Pharmacogn Rev.
;6:37-45.
. Madkor HR, Mansour SW, Ramadan
G. Modulatory effects of garlic,
ginger, turmeric and their mixture on
hyperglycaemia, dyslipidaemia and
oxidative stress in streptozotocinnicotinamide diabetic rats. Br J Nutr.
;105:1210-1217.
. Rana SV, Pal R, Vaiphei K, Singh K.
Garlic hepatotoxicity: safe dose of
garlic. Trop Gastroenterol. 2006;
:26-30.
. Nakagawa S, Masamoto K,
Sumiyoshi H, Harada H. Acute
toxicity test of garlic extract. J
Toxicol Sci. 1984; 9:57-60.
. Sumiyoshi H, Kanezawa A,
Masamoto K, Harada H, Nakagami
S, Yokota A, Nishikawa M,
Nakagawa S. Chronic toxicity test of
garlic extract in rats. J Toxicol Sci.
; 9:61-75.
. Akihisa T, Kamo S, Uchiyama T,
Akazawa H, Banno N, Taguchi Y
and Yasukawa K. Cytotoxic activity
of Perilla frutescens var. japonica
leaf extract is due to high
concentractions of oleanolic and
ursolic acids. J Nat Med. 2006;
:331-333.
. Borrelli F, Capasso R, Izzo AA.
Garlic [Allium sativum L.]: adverse
effects and drug interactions in
humans. Mol Nutr Food Res. 2007;
:1386-1397.
. Scharbert G, Kalb ML, Duris M,
Marschalek C, Kozek-Langenecker
SA. Garlic at dietary doses does not
impair platelet function. Anesth
Analg. 2007; 105:1214-1218.
. Sellers RS, Morton D, Michael B,
Roome N, Johnson JK, Yano BL,
Perry R, Schafer K. Society of
Toxicologic Pathology position
paper: organ weight
recommendations for toxicology
studies. Toxicol Pathol. 2007;
:751-755.
. Michael B, Yano B, Sellers RS, Perry
R, Morton D, Roome N, Johnson JK,
Schafer K, Pitsch S. Evaluation of
organ weights for rodent and nonrodent toxicity studies: a review of
regulatory guidelines and a survey of
current practices. Toxicol Pathol.
; 35:742-750.
. Bailey SA, Zidell RH, Perry RW.
Relationships between organ weight
and body/brain weight in the rat:
what is the best analytical endpoint?
Toxicol Pathol. 2004; 32:448-466.
. Aida Y, Kamata E, Nakadate M. A
study of the relationships between
exposure periods and no-effect
doses in repeated dose toxicity tests.
Eisei Shikenjo Hokoku. 1992;110:48-