Anti-Bacterial activity of total flavonoids of Portulaca oleracea L.
Keywords:
Flavonoids, Portuleca oleracea L, Antibacterial, Medicinal plantAbstract
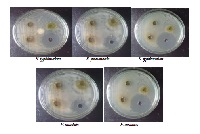
Flavanoids are uniquitous in photosynthesizing cells and are commonly found in fruit, vegetables, nuts, seeds, stems, flowers and wine. For centuries, preparations canting these compounds as the principal, physiologically active constituents have been isolated and used to treat human diseases. Portuleca olerace L is an important medicinal plant that has been used in traditional medicine. The present study was aimed to isolate the total flovanoids and to investigate an anti-bacterial activity of total flavonids extracted from aerial part of Portulaca olerace L. we have used five standard pathogenic bacterial strains like Pseudomonas aeruginosa, Salmonella typhimurium, Proteus mirabilis, Klebsiella pneumonia and Enterobacter aerogenes, among all the bacterial strains Salmonella typhimurium (14.33±0.2886) and Proteus mirabilis (17.16±0.0281) have shown maximum zone of inhibition for total flovanoids and remaining bacterial strains have shown moderate zone of inhibition when compared with control (20.66±0.2881). In case of bio-assay method Salmonella typhimurium was shown more sensitive by low turbidity of OD value (0.187) indicating most significant result. The Minimum inhibitory Concentration (MIC) of the total flavonoids isolated from Portuleca olerace L was tested at the concentration ranging from undiluted sample to 10mg/ml. the minimum inhibition concentration (MIC) for the total flavonoids for all tested bacterial strains was >10mg/ml. Experimental results supports that these flavonoids have antibacterial properties which helps in the developing antibacterial agents in the form of drugs for the therapy of infectious diseases caused by these bacterial pathogens.
References
. Jigna Parekh, Darshana Jadeja,
Sumitra Chanda. Efficacy of Aqueous
and Methanol Extracts of Some
Medicinal Plants for Potential
Antibacterial Activity, Turk J Biol,
; 29, 203-210.
. Seth S.D. and Bhawana. S. Medicinal
Plants in India of Medical Research,
; 120, 9-11.
. Lothfipour, F., H. Nazemiyeh, F. F.
Azad, N. Garaei and S. Arami.
Evaluation of antibacterial activities of
some medicinal plants from North
West Iran. Iran. J. Basic Med. Sci,
; 11: 80-85.
. Aboabo, O.O., S.I.Smith and F.O.
alude. Antibacterial effect of Edible
plant extract on E.Coli 0157, H7,
Part.J.Nutri, 2009; 5: 325-327.
. Davis J. inactivation of antibiotics and
the dissemination of registance
genes. Science, 1994; 264: 375-382.
. Service RF, Antibiotics that resist
registance. Science, 1995; 270: 724-
. Ahmad, I., Mehomood, Z. and
Mohammed, F. Screening of some
indian Medicinal plants for thire
antimicrobial properties. Journal of
Ethanopharmacology, 1998; 62(2),
-193.
. Mirjana, S.,B. Nanda and D. Valerija.
Variabality of Satureja cuneifolia ten
essential oils and their antimicrobial
activity depending on the stage of
development. Eur. Food Res.
Technol, 2004; 218:367-371.
. Ebana, R. U. B., Madunagu, B. E.,
Ekpe, E. D. and Otung, I. N.
Microbiological explanation of cardiaic
glycoside and alkaloids from Garcinic
kola, Barreria ocymoides, Kola nitida
and citrus aurantifolia. Journal of
applied Biotechnology, 1991; 71:398-
. Shariff, Z. U. Mordern Herbal Therpy
for Common Ailments. Nature
Pharmacy Series vol. 1, Spectrum
Book limited, Ibadan, Nigeria in
Association with Safari Books (Expert)
Limited, U. K, 2001. 9-84.
. Ivan.A. Ross. A Text book of
medicinal plants of the world.2003.
. Okwuasaba F, Ejika C, Parry O.
Effects of Portuleca oleracea L on
skeletal muscel in vitro. J
Ethanopharmacol, 1987; 27;55-63.
. RAdhakrishnan R, Zakaria mnm,
Islam MW. Neuropharmocological
actions of Portuleca oleracea L. v
sativa(Hawk) J Ethanopharmocol,
; 76; 171-176.
. Chan K, islam MW, Kamil M. The
analgenic and anti inflammatory effect
of Portuleca oleracea L subsp.
Sativa(haw) Celak. J
Ethanopharmacol, 2000; 73;445-451.
. Zargari A. Medicinal plants, Vol i. U
niversity Press; Tehran,1981; 218-
. Zannavi MA. Avicenna Conon, Vol 1.
Dar al-kotob al llmyah; Beirut,1999;
-406.
. Miller A G, Morris M. Plantas of
Dhofa, the southern Region of Oman.
Treditional Economical and Medicinal
Uses. The offece of the Advisor for
Conservation of Enveronmen,Diwan
of Court, Sultanate of Oman, 1988.
. Dermetzos C, Perdetzoglou DK.
Composition and antimicrobial studies
of the essential oils of Origanum
calcaratum Juss. and O.scabrum
Boiss. J. Essent. Oil Res. 2001;
:460–462.
. Prudent D, Perineau F, Bessiere JM,
Michel GM, Baccou JC, analysis of
essential oils of wild oregano from
Martinique (Coleus aromaticus
Benth.)- Evaluation of its
bacteriostatic and fungistaic
properties. J Essen Oli
Res.1995;7:165-173
. Delaquis PJ, Stanich K, Girard B,
Mazza G. Antimicrobial activity of
individual and mixed fractions of dill,
cilantro, coriander and eucalyptus
essential oils. Inter J Food
Microbiol.2002; 74: 10-109.
Nascimento, G.G.F., J. Locatelli, P.C.
Freitas and G.L. Silva. Antibacterial
activity of plant extracts and
phytochemicals on antibiotic resistant
bacteria. Braz. J. Microbiol, 2000; 31:
-256.
. Hassawi, D. and A.
Kharma. Antimicrobial activity of some
medicinal plants against Candida
albicans. J. Biol. Sci, 2006; 6: 109-
. Parekh, J. and S.
Chanda. Antibacterial and
phytochemical studies on twelve
species of Indian medicinal plants.
Afr. J. Biomed. Res, 2007;10: 175-
. Reuben, K.D., F.I. Abdulrahman, J.C.
Akan, H. Usman, O.A. Sodipo and
G.O. Egwu. Phytochemical screening
and in vitro antimicrobial investigation
of the methanolic extract of Croton
zambesicus Muell ARG stem bark.
Eur. J. Sci. Res, 2008; 23: 134-140.
. Forbes, B.A., D.F. Suhm and A.S.
Wissfeld. Baily and Scott's Diagnostic
Microbiology. 10th Edn., Mobsy Inc.,
London,1998.
. Bagamboula CF, Uyttendaele M,
Canada F, Daferera D, Unli GV,
Polissiou M, Sokmen A. Anttimicrobial
and antioxidative activities of essential
oils and methanol extracts of S.
cryptanha and S. multicaulis (Vahl.).
Food Chem.2004; 519-525.