ntioxidant and anti-proliferative activity of different solvent extracts of Casuarina equisetifolia needles
Keywords:
Casuarina equisetifolia, Antioxidant, Anticancer, Apoptosis, Caspase-3Abstract
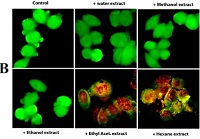
The context and purpose of the study: Plants that have been used for traditional medicines are very good sources of phyotochemicals. There are many plants like Casuarina equisetifolia, which are still unexplored for their medicinal properties. In the present study, we have elucidated the in vitro antioxidant and antiproliferative activity of different solvent extracts (both polar and non polar) of C. equisetifolia needles. Main findings: In vitro antioxidant activity of different solvent extracts of C. equisetifolia needles was studied by analyzing the total polyphenols, flavonoids, total antioxidant capacity and free radical scavenging activity. The polar solvent extracts showed significantly high amount total polyphenols, flavonoids, antioxidants and free radical scavenging activity compared to the non polar solvent extracts. The cytotoxic and apoptosis inducing activity of the different solvent extracts were analyzed on MCF-7 cells by MTT assay, acridine orange/ethidium bromide staining, DAPI staining and caspase-3 release. The polar solvent extracts did not show any growth inhibition in MCF-7 cancer cells. But the non polar solvent extracts are very good in inducing cell death by inducing apoptosis which involves DNA fragmentation and release of caspase 3. Using silica gel fractionation and RPHPLC analysis the active component present in non-polar solvent extracts was identified as ascorbic acid. Brief summary and potential implications: Our results indicated that the needles of C. equisetifolia are rich sources of antioxidants and also contain potential anticancer agents. Detailed study on the mechanism of action of purified compound on inhibition of cancer cell growth may provide some potential anticancer molecule from natural source.
References
. Hazra B, Biswas S and Mandal N.
Antioxidant and free radical scavenging
activity of Spondias pinnata. BMC
Compl. Alt. Med. 2008;9:63.
. Sacan O and Yanardag R. Antioxidant
and antiacetylcholinesterase activities
of chard (Beta vulgaris L. var. cicla).
Food Chem. Toxicol. 2010;48:1275-
. Chou ST, Chan HH, Peng HY, Liou MJ
and Wu TS. Isolation of substances
with antiproliferative and apoptosisinducing activities against leukemia
cells from the leaves of Zanthoxylum
ailanthoides Sieb. & Zucc.
Phytomedicine. 2011;18:344-348.
. Alshatwi AA. Catechin hydrate
suppresses MCF-7 proliferation through
TP53/Caspase-mediated apoptosis. J.
Exp. Clin. Cancer Res. 2010;29:167.
. Prakash D, Suri S, Upadhyay G and
Singh BN. Total phenol, antioxidant and
free radical scavenging activities of
some medicinal plants. Int. J. Food Sci.
Nutr. 2007;58:18-28.
. Chevallier A. “The Encyclopedia of
Medicinal Plants”. New York: Dk
publishing Inc. 1996.
. Slinkard K and Singleton VL. Total
phenol analyses: Automation and
comparison with manual methods. Am.
J. Enol. Viticol. 1997;28:49-55.
. Marinova D, Ribarova F and
Atanassova M. Total phenolics and total
flavonoids in Bulgarian fruits and
vegetables. J. Univ. Chem.
Technol.Metal. 2005;40:255-260.
. Prieto P, Pineda M and Aguilar M.
Spectrophotometric quantitation of
antioxidant capacity through the
formation of a phosphomolybdenum
complex: specific application to the
determination of vitamin E. Annals
Biochem. 1999;269:337-341.
. Chiu CY, Li CY, Chiu CC, Niwa M,
Kitanaka S, Damu AG, Lee EJ and Wu
TS. Constituents of leaves of
Phellodendron japonicum Maxim. and
their antioxidant activity. Chem. Pharm
Bull. 2005;53:1118–1121.
. Russo A, Izzo AA, Cardile V, Borrelli F
and Vanella A. Indian medicinal plants
as antiradicals and DNA cleavage
protector. Phytomedicine. 2008;8:125-
. Scudiero DA, Shoemaker RH, Paull KD,
Monks A, Tierney S, Nofziger TH,
Currens MJ, Seniff D and Boyd MR.
Evaluation of a soluble
tetrazolium/formazan assay for cell
growth and drug sensitivity in culture
using human and other tumor cell lines.
Cancer Res. 1988;48:4827-4833.
. Cohen JJ. Apoptosis. Immunol. Today.
;14:126–130.
. Tanious FA, Veal JM, Buczak H,
Ratmeyer LS and Wilson WD. DAPI
(4',6-diamidino-2- phenylindole) binds
differently to DNA and RNA: minorgroove binding at AT sites and
intercalation at AU sites. Biochemistry.
;31:3103-3112.
. Sakahira H, Enari M and Nagata S.
Cleavage of CAD inhibitor in CAD
activation and DNA degradation during
apoptosis. Nature. 1998;391:96-99.
. Finkel T and Holbrook NJ. Oxidants,
oxidative stress and the biology of
ageing. Nature. 2002;408:239-247.
. Kuate D, Etoundi OCB, Soukontoua
BY, Ngondi LJ and Oben EJ.
Comparative study of the antioxidant,
free radical savenging activity and
human LDL oxidation inhibition of three
extracts from seeds of a Cameroonian
spice, Xylopa parviflore (A.Rich.) Benth.
(Annonacea). Int. J. Biomed.
Pharm.Sci. 2011;5:18-30.
. Halliwell B, Aeschbach R, Loliger J and
Aruoma OI. (1995). The
characterization of antioxidants. Food
Chem. Toxicol. 1995;33:601-617.
. Rajkumar V, Guha G, Kumar AR and
Mathew L. Evaluation of antioxidant
activities of Bergenia ciliata rhizome.
Rec. Nat. Prod. 2010;4:38-48.
. Yu J, Liu H, Lei J, Tan W, Hu X and
Zan, G. Antitumor activity of chloroform
fraction of Scutellaria barbata and its
active constituents. Phytother. Res.
;21:817–822.
. Talib HW and Mahasneh MA
Antiproliferative Activity of Plant
Extracts Used Against Cancer in
Traditional Medicine. Sci. Pharm.
;78:33–45.
. Lee SH, Jaganath IB, Wang SM and
Sekaran SD. Antimetastatic effects of
Phyllanthus on human lung (A549) and
breast (MCF-7) cancer cell lines. PLoS
One. 2011;6:1-14.
. Pojarova M, Kaufmann D, Gastpar R,
Nishino T, Reszka P, Bednarski PJ and
von Angerer E. [(2- Phenylindol-3-
yl)methylene] propaneedinitriles inhibit
the growth of breast cancer cells by cell
cycle arrest in G2/M phase and
apoptosis. Bioorg. Med. Chem.
;15:7368–7379.
. Ahsan R, Islam KM, Musaddik A, and
Haque E. Hepatoprotective Activity of
Methanol Extract of Some Medicinal
Plants Against Carbon Tetrachloride
Induced Hepatotoxicity in Albino Rats.
Global J. Pharmcol. 2009;3:116-122.
. Harakeh S, Diab-Assaf M, Khalife JC,
Abu-el-Ardat KA, Baydoun E,
Niedzwiecki A, El-Sabban ME, Rath M.
Ascorbic acid induces apoptosis in adult
T-cell leukemia. Anticancer Res.
;27:289-298
. Kang JS, Cho D, Kim YI, Hahm E, Kim
YS, Jin SN, Kim HN, Kim D, Hur D,
Park H, Hwang YI and Lee WJ.
Sodium ascorbate (vitamin C) induces
apoptosis in melanoma cells via the
down-regulation of transferrin receptor
dependent iron uptake. J. Cell Physiol.
;204:192-197.