C-glycosyl flavones and a comparative study of the antioxidant, hemolytic and toxic potential of Jatropha multifida leaves and bark
Keywords:
Jatropha, Euphorbiaceae, Vitexin, Isovitexin, Antioxidant, FlavonoidAbstract
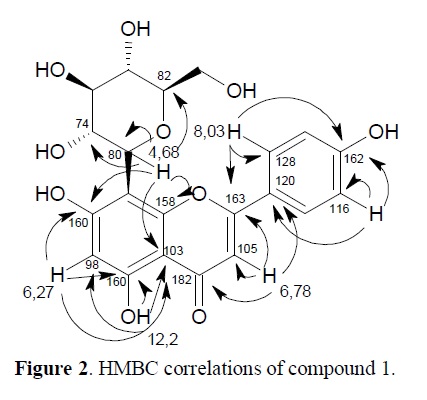
The ethyl acetate extract from Jatropha multifida (Euphorbiaceae) leaves yielded two C-glycosyl flavones. Their structures were elucidated through spectroscopic methods, including UV, IR, 1D and 2D NMR, and compared with the related known compounds. The structures of the two flavonoids were determined as Vitexin (1) and Isovitexin (2). The ethanol extracts of leaves and bark and their fractions did not interfere in the integrity of erythrocytes, not even 1 and 2. In the Brine shrimp lethality method, bark extracts showed greater toxic potential than the leaf extracts. Both flavonoids are not toxic. The Phosphomolybdenum and DPPH assays were used in order to investigate the antioxidant activity of both compounds and fractions of leaf and bark extracts. The ethyl acetate fraction of bark showed excellent activity, with IC50 17.23 μg/mL-1, equivalent to the standard values, Vitamin C and Rutin. Compounds 1 - 2 demonstrated good activity with IC50 values of 54.37 and 87.27μg/mL-1. In the Phosphomolybdenum test, the ethyl acetate fraction of bark showed 86.18% of antioxidant activity compared with Rutin, and the chloroform fraction of leaves, 103.29%. In all tests the bark extracts were more bioactive than the leaf extracts.
References
. Buch DR, Arantes AB, Campelo PM.
Verificação da atividade cicatrizante
do exudato de folhas de Jatropha
multifida. Rev Bras de Farmacogn.
;2:142-145.
. Hirota BCK, Trevisan RR, Dias JFG,
Miguel MD, Miguel OG.
Phytochemistry and biological
activities of genus Jatropha: minireview. Visão Acadêmica.
;11(2):103-112. URL:
http://ojs.c3sl.ufpr.br/ojs2/index.php/a
cademica/article/view/21374/14094.
. Kosasi S, Van Der Sluis WG, Labadie
RP. Multifidol and Multifidol glucoside
from the latex of Jatropha multifida.
Phytochemistry .1989;28(9):2439-
URL:
http://dx.doi.org/10.1016/S0031-
(00)98000-2.
. Ebuehi OA, Okorie NA.
Phytochemical screening and
quantification of flavonoids from leaf
extract of Jatropha curcas. Nig Q J
Hosp Med. 2009;19(4):200-5.
. Levin Y, Sherer Y, Bibi H, Schlesinger
M, Hayr E. Rare Jatropha multifida
Intoxication in Two Children. J Emerg
Med. 2000;19(2):173-175. URL:
http://dx.doi.org/10.1016/S0736-
(00)00207-9.
. Nogueira J B, Machado RDW.
Glossário de Plantas Oleaginosas e
Ceríferas II Euforbiáceas.
;vol2:p. 51-2.
. Das B, Ravikanth B, Reddy KR,
Thirupathi P, Raju TV, Sridhar B.
Diterpenoids from Jatropha multifida.
Phytochemistry. 2008;69:2639-2641.
URL:
http://dx.doi.org/10.1016/j.phytochem.
08.011.
. Das B, Reddy KR, Ravikanth B, Raju
TV, Sridhar B, Khan PU, Rao JV.
Multifidone: a novel cytotoxic
lathyrane-type diterpene having an
unusual six-membered A ring from
Jatropha multifida. Bioorg Med Chem
Lett. 2009;19:77-79. URL:
http://dx.doi.org/10.1016/j.bmcl.2008.
014.
. Meyer BN, Ferrigni NR, Putnam JE,
Jacobsen LB, Nichols DE, Mclaughlin
JL. Brine Shrimp: A Convenient
General Bioassay for Active Plant
Constituents. Planta Med.
;45:31-34.
. OMS, Quality Control Methods For
Medicinal Plant Material. 1998;p. 41.
. Flach J, Karnopp C, Corção G.
Biofilmes formados em matéria-prima
em contato com leite: fatores de
virulência envolvidos. Acta Sci Vet.
;33(3):291-296.
. Prieto P, Pineda M, Aguilar M.
Spectrophotometric quantitation of
antioxidant capacity through the
formation of a phosphomolybdenium
complex: specific application to the
determination of vitamina E. Anal
Biochem. 1999;269:337-341. URL:
http://dx.doi.org/10.1006/abio.1999.40
. Choi CW, Kim SC, Hwang SS, Choi
BK, Ahn HJ, Lee MY, Park SH, Kim
SK. Antioxidant activity and free
radical scavenging capacity between
Korean medicinal plants and
flavonoids by assay-guided
comparison. Plant Sci.
;163:1161-1168. URL:
http://dx.doi.org/10.1016/S0168-
(02)00332-1.
. Molyneux P. The use of stable free
radical diphenylpicrylhydrazyl (DPPH)
for estimating antioxidant activity.
Songklanakarin J Sci Technol.
;26(3):211-219.
. Mosquera OM, Correa YM, Buitrago
DC, Niño J. Antioxidant activity of
twenty five plants from Colombian
biodiversity. Mem Inst Oswaldo Cruz.
;102(5):631-634. URL:
http://dx.doi.org/10.1590/S0074-
. Agrawal PK. Carbon-13 NMR of
Flavonoids. Amsterdam: Elsevier;
. Mabry TJ, Markham KR, Thomas
MB. The Systematic Identification of
Flavonoids. Berlin: Springer; 1970.
. Silverstein RM, Webster FX, Kiemle
DJ. Identificação espectrométrica de
compostos orgânicos. 7th ed. Rio de
Janeiro: LTC; 2007;p.70-237.
. Peng X, Zheng Z, Cheng KW, Shan
F, Ren GX, Chen F, Mingfu W.
Inhibitory effect of mung bean extract
and its constituents vitexin and
isovitexin on the formation of
advanced glycation endproducts.
Food Chem. 2008;106:475-481.URL:
http://dx.doi.org/10.1016/j.foodchem.2
06.016.
. Moharram FA, Marzouk MS,
Haggag EG, El-Batran S, Ibrahim RR.
Biological examinationand novel
biflavone di-C-glycosides from
Jatropha multifida L. leaves. Planta
Med. 2007;73:048.
. Ongtengco DC. The in vitro
antibacterial activity of Jatropha
multifida Linn. latex against common
bacterial wound isolates. Acta Manila
Ser A. 1992;40(0):25-28.
. Aiyelaagbe O. Antibacterial activity
os Jatropha multifida roots.
Fitoterapia. 2000;72:544-546. URL:
http://dx.doi.org/10.1016/S0367-
X(00)00291-4.