A Convenient Mechanism for the Free Radical Scavenging Activity of Resveratrol
Keywords:
Resveratrol, antioxidant activity, tocopherol, ascorbic acid and butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA)Abstract
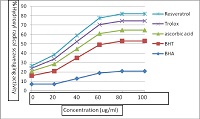
Resveratrol(3,5,4'-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced naturally by several plants when under attack by pathogens such as bacteria or fungi was evaluated for possible antioxidant and free radical scavenging activities. Different antioxidant tests were employed, namely, reducing power, chelating activity on Fe2+, free radical-scavenging, total antioxidant, superoxide radical scavenging, hydrogen peroxide scavenging and hydroxyl radical scavenging activities. In addition, the results were compared with natural and synthetic antioxidants, such as α- tocopherol, ascorbic acid, butylated hydroxytoluene(BHT), butylated hydroxyanisole (BHA) and trolox. Resveratrol exhibited a strong reducing power, chelating activity on Fe2+, free radical-scavenging, hydrogen peroxide scavenging and hydroxyl radical scavenging activities. Antioxidant activity of resveratrol increased with increased concentrations.Total antioxidant activity of resveratrol and both standards decreased in the order of α-tocopherol > resveratrol > trolox > BHA > BHT. This study showed that resveratrol exhibited antioxidant activity in all tests and could be considered as a source of natural antioxidants.
References
Farombi EO, Fakoya A. Free radical scavenging
and antigenotoxic activities of natural phenolic
compounds in dried flowers of Hibiscus sabdariffa
L. Mol Nutr Food Res 2005;49:1120–1128.
Gulcin I, Oktay M, Kufrevioglu O, Aslan A.
Determination of antioxidant activity of lichen
Cetraria islandica (L) Ach. J Ethnopharmacol
;79:325–329.
Gulcin I. Antioxidant and antiradical activities of Lcarnitine. Life Sci 2006;78:803–811.
Juntachote T, Berghofer E. Antioxidative
properties and stability of ethanolic extracts of Holy
basil and Galangal. Food Chem. 2005;92:193-202.
Hung LM, Chen JK, Huang SS, Lee RS, Su MJ.
Cardioprotective effect of resveratrol, a natural
antioxidant derived from grapes. Cardiovasc Res
;47:549-555.
Jang DS, Kang BS, Ryu SY, Chang IM, Min KR,
Kim Y. Inhibitory effects of resveratrol analogs on
unopsonized zymosan-induced oxygen radical
production. Biochem Pharmacol 1999;57:705-712.
Docherty JJ, Fu MM, Stiffler BS, Limperos RJ,
Pokabla CM,DeLucia AL. Resveratrol inhibition of
herpes simplex virus replication. Antiviral Res
;43:145-155.
Cheong H, Ryu SY, Kim KM. Anti-allergic action of
resveratrol and related hydroxystibenes Planta
Med 1999;65:266-268.
Fontecave M, Lepoivre M, Elleingand E, Gerez C,
Guittet O. Resveratrol, a remarkable inhibitor of
ribonucleotide reductase. FEBS Lett
;421:277-279.
Jang M, Cai L, Udeani GO, Slowing KV, Thomas
CF, Beecher CW, Fong HH, Farnsworth NR,
Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM.
Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes.Science
;275:218-220.
Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf
H, Harris GK, Shi X. Resveratrol scavenges
reactive oxygen species and effects radicalinduced cellular responses. Biochem Biophys Res
Commun 2003;309:1017-1026.
Oyaizu M. Studies on products of browning
reaction prepared from glucose amine. Jpn. J.
Nutr. 1986;44:307.
Decker EA. and Welch B. Role of ferritin as a lipid
oxidation catalyst in muscle food. J. Agr. Food
Chem. 1990;38:674-683.
Brand-Williams W, Cuvelier ME. and Berset C.
Use of a free radical method to evaluate
antioxidant activity. Lebensm. Wiss. Technol.,
;28:25-32.
Takashira M. and Ohtake Y. A new antioxidative
,3-benzodioxole from Melissa officinalis. Planta
Med. 1998;64:555-563.
Osawa T. and Namiki N. A novel type of
antioxidant isolated from leaf wax of Eucalpyptus
leaves. Agr. Biol. Chem., 1981;45:735-742.
Liu F, Ooi, VFC. and Chang ST. Free radical
scavenging activity of mushroom polysaccharide
extracts. Life Sci. 1997;60:763-772.
Ruch RT, Cheng SJ. and Klawnig E. Prevention of
cytotoxicity and inhibition of intercellular
communication by antioxidant catechins isolated
from Chineese green tea. Carcinogenesis,
;10:1003-1009.
Chung SK, Osawa T. and Kawakishi S. Hydroxyl
radical scavenging effects of species and
scavengers from brown mustard (Brassica nigra).
Biosci. Biotech. Biochem. 1997;61:118-123.
Bartosz, G. Druga twarz tlenu (The second face of
oxygen). Wydawnictwo Naukowe PWN,
Warzszawa(in Polish) 1995.
Bhandari MR and Kawabata J. Organic acid,
phenolic content and antioxidant activity of wild
yam (Dioscorea spp.) tubers of Nepal. Food Chem.
;88:163-169.
Bors W, Heller, W, Michel C and Saran M.
Flavonoids as antioxidants: determination of
radical-scavenging efficiencies. Methods Enzymol.,
;186:343–355.
Rice-Evans, C.A., Miller, N.J., and Paganga, G.,
Structure antioxidant activity relationship of
flavonoids and phenolic acids. Free Radi. Biol.
Med. 1996;20:933-956.
Sartor, L., Pexxato, E., Dell’Aica, I., Caniato, R.,
Biggin, S., and Garbisa, S., Inhibition of matrixproteases by polyphenols: chemical insights for
anti-inflammatroy and anti-invasion drug design.
Biochem. Pharmacol. 2002;64:229-237.
Yokozawa, T., Chen, C.P., Dong, E., Tanaka, T.,
Nonaka, G.I. and Nishioka, I. Study on the
inhibitory effect of tannins and flavonoids against
the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem.
Pharmacol. 1998;56:213-222.
Chung Y, Chen S, Hsu C, Chang C and Chou S.
Studies on the antioxidative activity of
Graptopetalum paraguayense E. Walther. Food
Chem., 2005;91:419-423.
Baumann, J., Wurn, G. and Bruchlausen, F.V.
Prostaglandin synthase inhibiting O2- radical
scavenging properties of some flavonoids and
related phenoic compounds. N-Ss Arch.
Pharmacol. 1979;308:R27-R39.
Cao, G., Sofic, E. and Prior, R.L. Antioxidant and
prooxidant behaviour of flavonoids: structure–
activity relationships. Free Rad. Biol. Med.
;22:749–760.
Sakanaka, S., Tachibana, Y. and Okada, Y.
Preparation and antioxidant properties of extracts
of Japanese persimmon leaf tea (kakinoha-cha).
Food Chem. 2005;89:569-574.
Juntachote, T. and Berghofer, E. Antioxidative
properties and stability of ethanolic extracts of Holy
basil and Galangal. Food Chem. 2005;92:193-202.