Beneficial effects of artichoke on liver phosphatidate phosphohydrolase and plasma lipids in rats fed by lipogenic diet
Keywords:
artichoke, hypercholesterolemia, liver triglyceride, plasma lipids, phosphatidate phosphohydrolaseAbstract
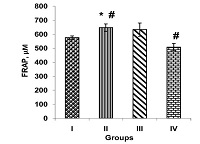
Artichoke (Cynara scolymus L.) is full of natural antioxidants and has a lipid-lowering effect. The aim of this study was to investigate the effect of artichoke on the liver phosphatidate phosphohydrolase, plasma lipid levels, plasma malondialdehyde, and plasma antioxidant in rats fed by lipogenic diet. Male rats were fed by standard pellet diet (group I), standard diet supplemented with 10% artichoke (group II), lipogenic diet (containing sunflower oil, cholesterol and ethanol) plus 10% artichoke (group III) and only lipogenic diet (group IV). On day 60 of the experiment, liver phosphatidate phosphohydrolase activity, liver triglyceride, plasma lipids, plasma malondialdehyde, and plasma antioxidant levels were measured. Phosphatidate phosphohydrolase activity, liver triglyceride, the ratio of total cholesterol to high density lipoprotein cholesterol, plasma total cholesterol and triglyceride levels were significantly decreased due to artichoke treatment in groups II and III compared to groups I and IV, respectively. Significant reduction in plasma malondialdehyde and significant elevation in plasma antioxidant power observed in groups II and III compared to groups I and IV, respectively. The results clearly indicated that artichoke can be useful for the reduction of phosphatidate phosphohydrolase activity and liver triglyceride. Also, artichoke has beneficial effects in the controlling of hyperlipidemia, abnormalities in lipid profiles and oxidative stress in hyperlipidemic regimes.
References
McKenney JM. Pharmacotherapy of
dyslipidemia. Cardiovasc Drugs Ther.
;15(5):413-422.
Laker MF. Cardiovascular disease
prevention: the new Joint British
Societies' guidelines. Ann Clin Biochem.
;43(Pt 5):335-339.
Gould AL, Davies GM, Alemao E, Yin
DD, Cook JR. Cholesterol reduction
yields clinical benefits: meta-analysis
including recent trials. Clin Ther.
;29(5):778-794.
Andersen C, Rayalam S, Della-Fera MA,
Baile CA. Phytochemicals and
adipogenesis. Biofactors. 2010;36(6):415-
Mulvihill EE, Huff MW. Antiatherogenic
properties of flavonoids: implications for
cardiovascular health. Can J Cardiol.
; Suppl A(26):17A-21A.
Küskü-Kiraz Z, Mehmetçik G, DogruAbbasoglu S, Uysal M. Artichoke leaf
extract reduces oxidative stress and
lipoprotein dyshomeostasis in rats fed on
high cholesterol diet. Phytother Res.
;24(4):565-570.
Joy JF, Haber SL. Clinical uses of
artichoke leaf extract. Am J Health Syst
Pharm. 2007;64(18):1904:1906-1909.
Shimoda H, Ninomiya K, Nishida N,
Yoshino T, Morikawa T, Matsuda H,
Yoshikawa M. Anti-hyperlipidemic
sesquiterpenes and new sesquiterpene
glycosides from the leaves of artichoke
(Cynara scolymus L.): structure
requirement and mode of action. Bioorg
Med Chem Lett. 2003;13(2):223-228.
Zapolska-Downar D, Zapolski-Downar A,
Naruszewicz M, Siennicka A,
Krasnodębska B, Kołdziej B. Protective
properties of artichoke (Cynara scolymus)
against oxidative stress induced in
cultured endothelial cells and monocytes.
Life Sci. 2002;71(24):2897-2808.
Juzyszyn Z, Czerny B, Pawlik A,
Droździk M. The effect of artichoke
(Cynara scolymus L.) extract on ROS
generation in HUVEC cells. Phytother
Res. 2008;22(9):1159-1161.
Wang M, Simon JE, Aviles IF, He K,
Zheng QY, Tadmor Y. Analysis of
antioxidative phenolic compounds in
artichoke (Cynara scolymus L.). J Agric
Food Chem. 2003;51(3):601-608.
Carman GM, Han GS. Roles of
phosphatidate phosphatase enzymes in
lipid metabolism. Trends Biochem Sci.
;31(12):694-699.
Fleming IN, Yeaman SJ. Purification and
characterization of N-ethylmaleimideinsensitive phosphatidic acid
phosphohydrolase (PAP2) from rat liver.
Biochem J. 1995;308( Pt 3):983-989.
Petersen KF, Shulman GI. Etiology of
insulin resistance. Am J Med. 2006;119(5
Suppl 1):S10-16.
Reue K, Phan J. Metabolic consequences
of lipodystrophy in mouse models. Curr
Opin Clin Nutr Metab Care.
;9(4):436-441.
Heidarian E, Haghighi B. Enzymological
characteristic of plasma membrane
phosphatidate phosphohydrolase (PAP2)
from rat liver. Iran J Sci Technol A.
;32:117-122.
Brindley DN. Lipid phosphate
phosphatases and related proteins:
signaling functions in development, cell
division, and cancer. J Cell Biochem.
;92(5):900-912.
Sciorra VA, Morris AJ. Roles for lipid
phosphate phosphatases in regulation of
cellular signaling. Biochim Biophys Acta.
;1582(1-3):45-51.
Gebhardt R. Inhibition of cholesterol
biosynthesis in primary cultured rat
hepatocytes by artichoke (Cynara
scolymus L.) extracts. J Pharmacol Exp
Ther. 1998;286(3):1122-1128.
Yanardag R, Peksel A, Yesilyaprak B,
Doger MM, Arisan-Atac I. Effects of a
combination of niacin and chromium(III)-
chloride on the skin and lungs of
hyperlipemic rats. Biol Trace Elem Res.
;103(3):249-260.
Friedewald WT, Levy RI, Fredrickson
DS. Estimation of the concentration of
low-density lipoprotein cholesterol in
plasma, without use of the preparative
ultracentrifuge. Clin Chem.
;18(6):499-502.
Norman SR. Preparation of lipid extracts.
In: John MI. (Ed.), Methods of
Enzymology. Academic Press, London;
;Vol. 14.
Haghighi B, Honarjou S. The effects of
hydrazine on the phosphatidate
phosphohydrolase activity in rat liver.
Biochem Pharmacol. 1987;36(7):1163-
Bradford MM. A rapid and sensitive
method for the quantitation of microgram
quantities of protein utilizing the principle
of protein-dye binding. Anal Biochem.
;72:248-254.
Ohkawa H, Ohishi N, Yagi K. Assay for
lipid peroxides in animal tissues by
thiobarbituric acid reaction. Anal
Biochem. 1979;95(2):351-358.
Benzie IF, Strain JJ. The ferric reducing
ability of plasma (FRAP) as a measure of
"antioxidant power": the FRAP assay.
Anal Biochem. 1996;239(1):70-76.
Frishman WH. Biologic markers as
predictors of cardiovascular disease. Am J
Med. 1998;104(6A):18S-27S.
Stone NJ. Lipid management: current diet
and drug treatment options. Am J Med.
;101(4A):4A40S-48S; discussion
S-49S.
Miller CA. Update on statins and other
lipid-lowering drugs. Geriatr Nurs.
;22(5):276-277.
Heidarian E, Jafari-Dehkordi E,
Seidkhani-Nahal A. Effect of garlic on
liver phosphatidate phosphohydrolase and
plasma lipid levels in hyperlipidemic rats.
Food Chem Toxicol. 2011;16:1110-1114.
Wider B, Pittler MH, Thompson-Coon J,
Ernst E. Artichoke leaf extract for treating
hypercholesterolaemia. Cochrane
Database Syst Rev. 2009;7(4):CD003335.
van Herpen NA, Schrauwen-Hinderling
VB. Lipid accumulation in non-adipose
tissue and lipotoxicity. Physiol Behav.
;94(2):231-241.
Elabbadi N, Day CP, Gamouh A, Zyad A,
Yeaman SJ. Relationship between the
inhibition of phosphatidic acid
phosphohydrolase-1 by oleate and oleoylCoA ester and its apparent translocation.
Biochimie. 2005;87(5):437-443.
Jemai H, Fki I, Bouaziz M, Bouallagui Z,
El Feki A, Isoda H, Sayadi S. Lipidlowering and antioxidant effects of
hydroxytyrosol and its triacetylated
derivative recovered from olive tree
leaves in cholesterol-fed rats. J Agric
Food Chem. 2008;56(8):2630-2636.
Sudhahar V, Kumar SA, Varalakshmi P,
Sundarapandiyan R. Mitigating role of
lupeol and lupeol linoleate on hepatic
lipemic-oxidative injury and lipoprotein
peroxidation in experimental
hypercholesterolemia. Mol Cell Biochem.
;295(1-2):189-198.
Ondrejovičová I, Muchová J, Mišľanová
C, Nagyová Z, Ďuračková Z.
Hypercholesterolemia, oxidative stress
and gender dependence in children.
Prague Med Rep. 2010;111(4):300-312.
Lykkesfeldt J. Malondialdehyde as
biomarker of oxidative damage to lipids
caused by smoking. Clin Chim Acta.
;380(1-2):50-58.
Juzyszyn Z, Czerny B, Myśliwiec Z,
Pawlik A, Droździk M. The effect of
artichoke (Cynara scolymus L.) extract on
respiratory chain system activity in rat
liver mitochondria. Phytother Res.
;24 Suppl 2(24):S123-128.
Brown JE, Rice-Evans CA. Luteolin-rich
artichoke extract protects low density
lipoprotein from oxidation in vitro. Free
Radic Res. 1998;29(3):247-255.
Pérez-Garcia F, Adzet T, Canigueral S.
Activity of artichoke leaf extract on
reactive oxygen species in human
leukocytes. Free Radic Res.
;33(5):661-665.
Jiménez-Escrig A, Dragsted LO,
Daneshvar B, Pulido R, Saura-Calixto F.
In vitro antioxidant activities of edible
artichoke (Cynara scolymus L.) and effect
on biomarkers of antioxidants in rats. J
Agric Food Chem. 2003;51(18):5540-